How To Memorize The Strong Acids and Strong Bases
Vložit
- čas přidán 27. 07. 2024
- This chemistry video tutorial explains how to memorize the 7 strong acids and strong bases. Strong acids dissociate completely where as weak acids dissociate partially. Strong Bases are usually soluble compounds that release hydroxide ions into solution.
Acids and Bases - Introduction: • Acids and Bases - Basi...
The 7 Strong Acids to Memorize:
• How To Memorize The St...
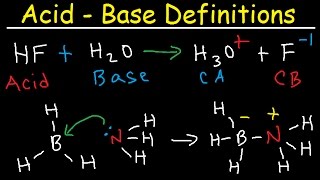
Conjugate Acid-Base Pairs:
• Conjugate Acid Base Pa...
pH and pOH Calculations:
• pH, pOH, H3O+, OH-, Kw...
Estimate The pH Without a Calculator:
• How To Calculate The p...
_______________________________
Autoionization of Water - Kw:
• AutoIonization of Wate...
Which Acid Is Stronger?
• Acid Base Strength - W...
Acidic, Basic, & Neutral Salts:
• Acidic, Basic, and Neu...
pH of Weak Acids:
• pH of Weak Acids and B...
Buffer Solutions:
• Buffer Solutions
_________________________________
Polyprotic Acid Base Equilibria:
• Polyprotic Acid Base E...
Acid Base Titration Curves:
• Acid Base Titration Cu...
Acids and Bases - Practice Test:
• Acids and Bases Review...
Ksp - Molar Solubility & Ice Tables:
• Ksp - Molar Solubility...
Complex Ion Equilibria:
• Complex Ion Equilibria...
___________________________________
Gibbs Free Energy, Entropy & Enthalpy:
• Gibbs Free Energy - En...
Entropy - 2nd Law of Thermodynamics:
• Entropy - 2nd Law of T...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tutor.net/chemistry...







Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Full-Length Videos & Exams: www.patreon.com/MathScienceTutor/collections
Next Video: czcams.com/video/OP6RKqSp1Xw/video.html
honestly might drop out and homeschool myself with ur videos
spoke too soon, my friend. We're all learning at home
Hahahahaha
exactly
Yeah
dude your saving my grade. thank you sooo much, your lessons actually make sense
Don't scroll comments
And concentrate what sir is teaching 😂😂
Bro is single handedly saving me for my chem exam.
you are really making my study easier. :D
I'm in love w this dude frfr
Made 5 years ago, and yet this still carries me through Chem 2. TYSM!
tysm!! honestly you r saving grades
awesome. thank you so much! love from Brazil
You are the only my best teachers thank you
Hmm I guess LSD is a different type of “strong” acid. This guy carried me through calc 1-3, physics 1 and 2, statics if I’m not mistaken, and now chemistry. The Organic chemistry tutor is my god. The Organic chemistry tutor is my religion. He is omnipresent
Haha see what u did at the end with “this is just a basic introduction”
I thought that the conjugate of a weak acid was a strong base? Is that wrong? Or is it the addition of the salt that causes the strong conjugate base to become weaker?
Thank you
Love from Ethiopia🇪🇹
I owe this guy my tuition
Thanks ❤
U r truly my chem teacher
You're the best!
Dude, you are simply the best👩🎓🤝
THANKYOU-so-so-much-it-helped-me-so-much-in-ionic-equilibrium
How about hydronium ion? Is it weak or strong acid?
Man you r great dude just keeps coming
great video. I was just wondering what does pka stands for?
pKa is an acid dissociation constant used to describe the acidity of a particular molecule. Its value is directly related to the structure of the given compound.
was Ca(OH)2 on the list??
Ur videos are better than my tutors lol
Sir, we find salts by looking at their formation right?
Like, strong basic+strong acidic is neutral
Strong base+weak acid is basic
Strong Acid + weak base is acidic
Weak acid + weak base is neutral
Right??
Pretty much but weak acid and weak bases dont react with each other
they do show maximum hydrolysis whereas SA and SB do not show hydrolysis@@samibs7215
Thank god i finally understand
thank you for this
Got chem test tomorrow, wish me luck bois
Best if luck
Of*
It was helpful.❤
What I hate ab regular science/physical science is my teacher leaves out really helpful tips for the sake of making it “easier” of simplifying it. When in reality I wouldn’t mind having to memorize this stuff bc if makes everything Sm easier
6:48 Can someone please explain? Shouldn't it be the opposite?? Shouldn't the conjugate base of a weak acid be a strong base?
Your vedios are easy to understand,
great video
Hey sir no pressure, but can you please make a video on period 3 elements, please?
hi i know your comment is from like 2 yrs ago and this is a chem vid but i just wanted to say i like your ningguang profile pic
@@kawaroki hihi, I don’t even remember saying this 😭 thank you for giving her love, shes gorgeous and therapeutic
@@eri4232 Im bringing you back to this comment section another year later.
just group 1 and 2 metals for bases right?
Me and my roommates only refer to this guy as “Science Jesus” here to save you from all your terrible preparation sins
Your rewards are great trust me
what is the relation between nano volume and the hardness
I cannot thank you enough
Are you planning to create video on Probability Dice problems? Please sir I need your respond.
zaki Abdihukun look up ‘probability’ on khan academy. Should be on CZcams. :)
@@thor-robertj2100 Thanks I prefer this chennel.
What is pka
thankyou sir
Please can you clarify this part of the video 6:42 "the conjugate of aweak acid would be a weak base" ?
Same problem that I noticed! I got a little bit confused with this one >_
I have the same problem too@@Lila_DAYBREAK
Love from india 🇮🇳
7:15 isn't the conjugate of weak acid a strong base?
great now i am confused
i think its basic but not neccerally strong
something i learned in chem class is to look for both the base and acid conjugates
for example NaF conjeagtes are NaOH( stong base) and HF(weak acid) so NaF is basic salt because the base is stronger
strong base + strong acid --> neutral
strong base + weak acid --> basic
weak base + strong acid --> acidic
weak base + weak acid --> neutral
though i am not sure of their strength as acidic and basic salts so will do more research
Yh...it's been a year now since you commented...but I think the conjugate base of a weak acid should be a strong base....It's getting me too confused when I got to some part of the video after I was trying to make some reference....I think organic chemistry tutor, you should speak concerning that side well for us to make us clarified....thank you
HBrO4 is a strong or weak acid
haha, BASIC Introduction, I get it
Lol
Can someone explain at 9:34 how NaF turns into HF? I'm not understanding how the Na disappears...thank you!
It does not turns into HF, HF is the conjugate acid of NaF(strong base) as he mentions the conjugate of strong base/acid is a weak base/acid and vice versa
The conjugate of NaF(strong base) is HF(weak acid)
@@labeebasari380 NaF is a weak base
@@labeebasari380 How is NaF a strong base, isn't it weak? Wouldn't NaOH be strong, and NaH be weak?
love from Ethiopia
is ammonium an acid or a base cause in some vids they said acid and others say base
Maybe both. He said it's both.
what is the meaning of pka?
pKa = -log base 10 (Ka)
It is basically how much the acid will dissociate when added in water.
What about H3PO4
Isn't it a strong acid as well??
C20H25N3O is the strongest of them all.
@@Hellcommander245 I didn't mean which is stronger
I meant that H3PO4 is a strong acid as well but he didn't mention it in the video.
Rania Ashraf nope its pkA is 2, so above -1
Rania Ashraf Mr. Crabs is kidding, the pKa of his 'acid' is 7.8, so not an acid. it is however extremely potent, lifechangingly so!! 🤩🤯🤩🤯🤩🤯
@@richardwiersma I don't see no Mr. Crab here
life saver
This video is 2 semesters too late!
Isn’t the conjugate base of a weak acid a strong base?
When he says "now let's talk about" he sounds like "let's suck up out"
Trueee
What’s pka
Pka = -log(ka)
@@jeremiahv6498 what's "ka"?
@@jeremiahv6498 I've never heard of this
Maybe we use a different format for the same thing
Ikonik_Konklusion ¿ Ka is the equilibrium constant for a acid.
Kb is the Equilibrium constant for a base.
When ever you see “P” that basically means “-log”
I paid 3,400 to take high school classes I didn't take when I was a kid. should've just watched your videos -.-
See what you did there "this is just a basic introduction" hehehhehehhehehee
🇪🇹Ethiopian legends show ur self( like this comment)
What grade are you in 🤷♂️
❤
Ethiopia
U r truly my chem teacher