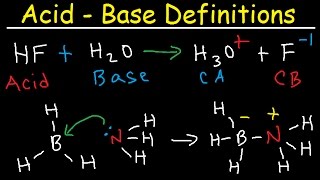
Acidic, Basic, and Neutral Salts - Ionic Compounds
Vložit
- čas přidán 28. 07. 2024
- This chemistry video explains how to determine if an ionic compound or a salt is acidic, basic, or neutral. Acidic ions include the ammonium ion and metal cations such as Al3+ and Fe3+. Basic ions include the conjugates of weak acids. Neutral ions include the alkali metal cations and conjugates of strong acids.
Acids and Bases - Introduction:
• Acids and Bases - Basi...
The 7 Strong Acids to Memorize:
• How To Memorize The St...
Conjugate Acid-Base Pairs:
• Conjugate Acid Base Pa...
pH and pOH Calculations:
• pH, pOH, H3O+, OH-, Kw...
Estimate The pH Without a Calculator:
• How To Calculate The p...
_______________________________
Autoionization of Water - Kw:
• AutoIonization of Wate...
Which Acid Is Stronger?
• Acid Base Strength - W...
Acidic, Basic, & Neutral Salts:
• Acidic, Basic, and Neu...
pH of Weak Acids:
• pH of Weak Acids and B...
Buffer Solutions:
• Buffer Solutions
_________________________________
Polyprotic Acid Base Equilibria:
• Polyprotic Acid Base E...
Acid Base Titration Curves:
• Acid Base Titration Cu...
Acids and Bases - Practice Test:
• Acids and Bases Review...
Ksp - Molar Solubility & Ice Tables:
• Ksp - Molar Solubility...
Complex Ion Equilibria:
• Complex Ion Equilibria...
___________________________________
Gibbs Free Energy, Entropy & Enthalpy:
• Gibbs Free Energy - En...
Entropy - 2nd Law of Thermodynamics:
• Entropy - 2nd Law of T...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tutor.net/chemistry...








Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Full-Length Videos & Exams: www.patreon.com/MathScienceTutor/collections
Next Video: czcams.com/video/kJTCuRSeh6g/video.html
Just incase anyone doesn't understand. Refer to the table he provided first of all and then if given a compound, lets say NH4NO3 for example, you can seperate it into its respective ions (NH4+ and NO3-) and then always cancel out the neutral one (in this case, NO3- is neutral because it is the conjugate for a strong acid). If both ions are neutral according to the table he provided, then it means overall the compound is neutral. Remember to always cancel the neutral ion and then the remaining ion is what you use to determine whether the compound is acidic, basic or not (NH4+ is acidic and NO3- was cancelled out, therefore NH4NO3 is an Acidic compound). I hope this helps someone🙂.
This was one of the few times I had trouble understanding one of his videos, however your comment simplified things so well, thank you!
@@stephen1558 I am glad you found it helpful, you are very welcome🤗.
Very helpful
@@xdoubt4004 ❤
I find it very helpful now i understand it more now 😊😊😊😊😊 thinkyouberrymuch
Dear Organic Chemistry Tutor,
Yesterday I stayed home and ate beans and rice. I saved a few bucks so I can support you because I love you. Expect some revenue from a fanny pack sale soon.
Sincerely yours,
Organic Chemistry Student
Thanks for a good year of AP Chem cuz my teacher couldnt really teach properly
The consistency of how well you put these explanations together is immaculate.
I love your vids
thats what comprehensive and deep understanding will do for ya.
You have been my replacement chemistry professor this semester! Thanks for making these uber clear and easy-to-understand videos!
It's so good with so many examples it's just what I needed. I am really thankful and I really appreciate your help..☺️
Thank you for dedicating your time to helping others with chemistry. I see many college professors are lacking in their teaching skills and you fill in the gaps. I really hope you are an instructor/professor. Any university would be very lucky to have you!
Dude helps with more than chem. He got me through pre calc, calc 1, and basic circuits
thanks so much for the wonderful and consistent videos they help me a lot :)
Great video as always
i literally luv u sm i rly apreciate evth u do idk what id do wuthout this channel i hope u get great things in life
Thanks man, this will be very helpful for my upcoming che exam
u are truly a top G my friend
Thanks you really helped me out!
Hi
It really helps me for 7th grade this year in 2020!
U r nation?
can we use this method to find out a redox reaction is acidic or basic
hello! could you do a video on Registers and Flip Flop[s?
how do you do it without the table? aka, how would you know those ions were basic/acidic/neutral?
But what about putting Beryllium chloride in water? When the universal indicator is added to it, it is red aka a strongly acidic solution?
Thanks
what about Al(OH)3 or Al2O3? Here both strong acid and strong bases are present.
If we are following his method in 6:45 shouldn’t co2 be a base
I miss teaching Chemistry
How to know which gas is real or ideal like H2, He,Co2,ch4??
Hi
gases behave like ideal gases at high temperatures and low pressures
@@GiveMeThatSwordPower Otherwise all gases r real, but at high temperature and low pressure they become ideal.
@@godhelpme8977 hi
@@shalinitiwari119 they "act" ideal - as pressure increases these gases have either a volume less than 22.4 or a volume greater than 22.4. for example co2 has a volume less than 22.4 as pressure increases (from near 0), so it starts to act more like a real gas
HClO3 is a strong acid** 1:43, first mistake I found in your channel after 4 years of watching lol
You're correct
Ty sir
But where is organic chemistry
Actually chloric acid, HClO3, is a strong acid.
9:57 barely can tell that is copper. looks more like C O
it's good.But sir i am preparing for Telangana EAMCET exam.Sir can you do a video by covering important syllabus for EAMCET by previous paper analysis....or by your own.
I still don't understand please,😔😔
I thought HClO3 was a strong acid....
Yes it's a strong acid
wish i watched this before my exam
I don't understand
Refer to the table he has given