16.1 Introduction to Acids and Bases | General Chemistry
Vložit
- čas přidán 27. 06. 2024
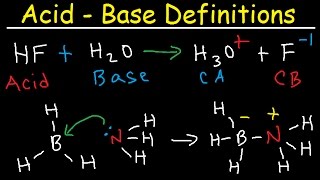
- Chad provides an introduction to acids and bases beginning with three common definitions for acids and bases: the Arrhenius acid and base, the Bronsted-Lowry acid and base, and the Lewis acid and base. The differences between the definitions are discussed with examples that demonstrate these differences. An Arrhenius acid is an H+ donor in water which increases the concentration of H3O+. An Arrhenius base is an OH- donor in water which increases the OH- concentration in water. A Bronsted-Lowry acid is an H+ donor, and a Bronsted-Lowry base is an H+ acceptor and solvents besides water are envisioned. Finally, a Lewis acid is an electron acceptor, and a Lewis base is an electron donor, and acid base reactions that only satisfy Lewis' definition are explained. Also covered is how to identify conjugate acid-base pairs using the Bronsted-Lowry acid and base definitions. Finally, strong acids and bases are defined and a list of strong acids and a list of strong bases are provided.
I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at www.chadsprep.com/chads-gener...
If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course (free trial available) at www.chadsprep.com/genchem-you...
00:00 Lesson Introduction
00:26 Arrhenius Acids and Bases
03:31 Bronsted-Lowry Acids and Bases
06:50 Lewis Acid and Base
13:16 Conjugate Acid-Base Pairs
23:05 Strong Acids and Strong Bases
www.chadsprep.com/
courses.chadsprep.com/pages/p...








You made my day when you mentioned HF can dissolve dead bodies like in Breaking Bad
Yeah Science!
You just took my love for chemistry to a new high,thank you for the lesson Chad
That is great to hear - you're welcome!
You made comprehension of my lectures easier. Thank you, Chad; you are the GOAT.
You're welcome - and Thank You!
You are literally the reason as to why I understand chemistry. My professor has made these concepts out to be so difficult and you make it so simple and explain it so well! You are a LIFESAVER! Definitely coming back for Organic chem help! Thank you!
Glad the channel is helping you - Happy Studying!
You are amazing. And hopefully your channel will grow.
For now I will do my part by sharing it to my mates
Thank you - Appreciate that!
This channel has become my go-to for Chem 2
Glad it is, FFFH - Happy Studying!
Your videos are so helpful. Thanks!
You're welcome, F.P. - and thank you!
Beautiful explanation! Cant wait for next video to come out
Awesome! It's coming in the morning and another Thursday morning.🙂
@@ChadsPrep please do a video on buffers and titrations
Hey Dhruv! If you explore the channel you'll see an old Gen Chem playlist with buffers and titrations covered in chapter 17. This lesson is part of an updated playlist and I'll be remaking and releasing these analogous chapter 17 videos week after next. But the old ones are also embedded in a free course on my site often with some extra content and organized with a dropdown menu that you might appreciate. You'll find the lessons here:
www.chadsprep.com/chads-general-chemistry-videos/buffer-chemistry/
www.chadsprep.com/chads-general-chemistry-videos/titration-curves/
www.chadsprep.com/chads-general-chemistry-videos/titration-calculations/
Lots of extra content for titration calculations on that last page. Hope this helps!
I think you are by far the best Professor I've come across. Thank you for your work!
Greetings from Cyprus!
You're welcome and Thank You from USA!
I am a chemistry student and your explanations helped me so much. Thank you!
Excellent!
wish you were my chem professor... Thanks for this lesson!
You're welcome!
Yehey! I found the best explanation about these concepts. I haven't taken Chem in my entire life, so thank you soo much. 🙏👍😊
You're welcome - glad it helped!
Thank you so much, Chad.
You're welcome.
I wish I could have you as my professor. Out of all the chemistry professors I have delt with. you seem informed, organised, and able to communicate effectively...As a student that is all i am asking for.
Thank you!
Great work sir.
Thank You
NIce simple explanation! Thank you!
You're welcome!
Thank you for your explanation. It has saved me so much time reading books and trying to figure out how to analyze the information.
You're welcome!
love the video + the shirt! thanks for all your help :)
Thank you + thank you - You're Welcome!
Thank you so much for the amazing lesson! I was just wondering if you have any plans on teaching inorganic or analytical chemistry and making videos in those fields? Because you're the best teacher i've EVER had
your videos save my AP chem grade you make it so logical and simple!! God bless you
Glad to hear that and Thank You!
Thank you for explaining things so well, so thoroughly:)
Glad it was helpful!
Absolute legend, thank you so much!!!
Very welcome!
Super helpful! Thank you!
Very welcome!
Thank you Chad!
You're welcome!
not LSD 😭 this man is gold
Glad you think so!
thank you
You're welcome
What is the difference between MCAT prep video and this?
I think more examples would have done it but it was great
Thanks.
you’re everything
Glad the channel/videos are helping you!
@@ChadsPrep In my prayers you’re 🤍
@@mahra855 Thank you.
Im back to thank you again Chad :) the breaking bad comment made me check comments so..
Thank you for this ❤
You're welcome and Thanks!
Hi Chad, can you explain why acids only donate 1 H+ to become a base? For example, why isn't the base of acid CH3COOH then CH2-COO- for example, or why not donate all the H to become C(3negative)COO-? Or NH4+ then N(4negative) instead of NH3?
Not all of those H's in CH3COOH are acidic. The H-C bonds are not polar so it is not easy to break the bond and lose the H. The O-H bond in a carboxylic acid is much weaker due to high polarity of the O-H bond and further charge delocalization from the carbonyl group which stabilizes the conjugate base CH3COO- making that H acidic (dissociation occurs in solution). In order to break the C-H bond we would have to put energy in, it wouldn't freely dissociate in solution. Hope that helps!
@@ChadsPrep yes that helps, thank you
But did you mean to say the C-H bonds are not polar? Instead of H-C?
sir may you please assist in solving the following problem
A 25ml sample of 0,10M sodium benzoate is titrated with 0,10 M of HCl what is the pH of after the addition of of 32,0ml of HCl (kb of C6H5CO2- =1,6X10^-10)
I cover titrations in this playlist in chapter 17. I specifically cover weak acid/strong base and weak base/strong acid in this lesson: czcams.com/video/3Y9TaxwcQGs/video.html
Tune in around 21:16 for how to calculate the pH when you are past the equivalence point. Hope this helps!
19:28 this part caught me off guard because the Super Bowl just happened and this video was filmed one year ago
What timing!
this man is literally Heisenberg. Didn't know he was still living.
Ssshhhhh - no one needs to know!
What does your shirt say?
Trust in the Lord with all your heart and lean not on your own understanding. In all your ways acknowledge Him and he shall make your paths straight. Proverbs 3:5+6