Acids and Bases - Basic Introduction - Organic Chemistry
Vložit
- čas přidán 29. 06. 2024
- This video provides a basic introduction into acids and bases with reference to organic chemistry. It explains how to write acid base reactions as well as how to determine the conjugate acid and the conjugate base. It covers the brownsted lowry definition of acids and bases as well as the lewis version. This video also explains how to determine if an acid base reaction is product favored or reactant favored.
Access The Full 1 Hour 20 Minute Video:
/ mathsciencetutor
Direct Link to The Full Video:
bit.ly/3k9vuX6
Organic Chemistry PDF Worksheets:
www.video-tutor.net/orgo-chem...
_______________________________________
Join The CZcams Membership Program:
bit.ly/46xaQTR
Full 1 Hour 20 Minute Video on CZcams:
• Acids and Bases - Basi...






Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Full-Length Exams & PDF Worksheets: www.patreon.com/MathScienceTutor/collections
Get The Full 1 Hour 20 Minute Video: www.patreon.com/MathScienceTutor
Direct Link to The Full Video: bit.ly/3k9vuX6
your so cool
My tuition should go to you instead of school
That’s what I am saying bra
I learn more math from you chem videos than from my math teacher😂
I agree
Exactly
I'm always here every time I have exams/tests etc. And now I think I'm falling for u
Bro?
exactly wat i do too
@@therealminatonamikaze188 socks on it's all good bro
@@therealminatonamikaze188 no sus
Lmaoooo
I got an A in Calc 2 thanks to this guy!
Now I watch most of his videos just to support. Thanks for everything!
And we let the ads run, so he can monetize! He is the best!
I just want to say you’re a very Genius man!! I got “B” grade in organic chemistry which was a real pain for me after God willing then by watching your lessons!!! You taught me more than my previous professors in chemistry department. So thank you and God bless you👏
Definitely worth you're weight several times over in gold. When I give up on my teachers you're videos remind me of the love I still have for the subject.
After years of watching your videos, I’m finally getting to your organic chemistry stuff lol. Thanks for the content!!
sending a massive thank you your way for helping me understand concepts that i seriously doubt i could have a grasp of otherwise. im currently in a chemistry II class and although i love chemistry very much i have a hard time grasping the concepts. your videos have been such a massive help to me through the last few years with both math and chemistry. you are amazing and i hope that you are having a lovely day/night
This series has helped me grasp acid base equilibria concepts better than when I had learnt it in my school. THANK YOU!
Before i watch this, I'd like to say thank you so much! It's a big part of my journey. May God bless you always.
After so many years of following, admiring and learning, I am so blessed to be the first comment. You have inspired my own channel and I I am very thankfull for everything you have contribute for the science community. God Bless You!
Ever considered reading ikea instructions on an audio tape? Also your the man.
Completely delightful! Thank you!!!
I'm not even a science student yet here I'm procrastinating over stuff like this. Pretty interesting.
We're literally going over this rn, thank you!!! ❤ Amazing timing!!
you are doing amazing work - thanks
my prof cant even explain things like you! thank you for saving us!
The only channel on youtube i will watch full ads on willingly
Thanks for the hard work!!!
I pray that God give you long life!!
Very helpful.. I am able to learn college thing in 10th grade...
I really like your teaching. You are the cause of me learning calc 1 and trig in class 9.
Thank ☺you so much
Thank you for helping me in my studies. Now I'm considering BS Biochemistry as my course.
i just bought your membership today ! i have ochem summer session my first test is wednesday. you are saving my life just so you know ;) I just want to make sure im clear the WA will be more stable ?
Thanks. It really helps 👍👍
your videos are so helpfull thank you so much
Thanks for everything you helped me a lot💜
Thank you this was so useful
JG you the best, thank you 😊
everything i dont understand at school i watch here and understand
Hiii! Can you create a video about the whole of reaction kinetics and redox equilibria pleaseeee xxx
Hey I am a class 11 Indian student... In which standard are you learning this?
My number one Teacher 🙏⚡
thank you so much!!!
Love the videos! Your the best!
He is, truly!
You are the best! But I is hard 8:05. I need to study more.
I hope Elon Musk will notice this youtube channel and donate funds to grow more...
Amazing
I love the you teach
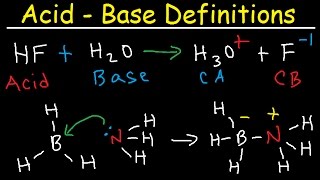
For the last example in the video, the reaction between F- and CH3OH is reactant favored because the pKa of CH3OH is greater than HF (thus CH3OH is weaker acid). Why do we not consider pKb in determining which side is favored?
pKa and pKb are related: 14 = pKa + pKb. You can use either set or even a blend of pka and pkb values to determine the direction of the equilibrium. The smaller the pka, the greater the pkb, but since the conjugate pair is on the other side, your answer stays the same.
It's a year now😮...but I think we were given pka of the acid (Ch3OH) and it's conjugate acid (HF)....so I'm not sure there would be a need to be comparing the pkb of their conjugate bases....you might as well end up confusing your self...I was almost confused and had to refer back to the video ...I now realised what he was doing😂....I've gotten it now more clearer...but I think ...you can also reference to see it...thanks😊 25:40
Thank you so much
My tuition should go to you instead of my school😂❤❤
Yay I can just watch this for my exams instead of reading the book
Same
same here
My lectures are really making me hate my modules... They cover nothing.... I come back home to watch your videos that give me hope if not for your channel I would have quit university
RCT 2021 cutiiie! 💖
At 25:45- why did B become -1. Shouldn’t B be -2 as it has 5 Ve’s (in BH3) and should have 3?
28:18 Doesn't the universe tend towards disorder and entropy? If so, shouldn't the reaction go towards the unstable side?
I came here just to like the video
What about the structure tells us which is the bronsted acid/ base? You don’t say why HF is acid or why NH3 is base. Please clarify, I would rather not have to memorize weak acids/ bases.
Even UNACADEMY doesn't have this much subscribers 😂
In this process( HF + H2O ) becomes( F- + H3O +) and it is said to be a reversible reaction!
Why does HF + H2O have to become F- + H3O + and reverse back to HF + H2O ?
It can just not form F- + H3O !!
Please reply 🙏 🙏🙏
I know it is a silly question but I just wanna know!!
because F- conjugate base of strong acid HF which will dissociate completely in water.therefore is no way for ths reaction to be reversible reaction.it's done in one direction.
HF is not strong @@hakimalaourai276
What is the meaning of pa?
So I'm having a problem. How do I know which acid is stronger without a given pka or ka.
basic strong acids: HCl, HBr, HI, HNO3, H2SO4, HClO4, HClO3 basic weak acids: HF, NH4, CH3COOH, H2CO3, HNO2, H2SO3 you should memorize all of them
@@MrChAcHa96 Yes but what makes them strong acids and strong bases? Is there a consistent rule that can be followed so that we can potentially understand what's going on?
@@noakuu393 actually chemists discovered them so you don’t need to mess your brain to understand properly what’s strong acid or not. But if you want the explanation is the following: take for example HI (pka-10) and HBr (pka-9) both strong acids, remind that stronger the acid weaker the conjugate base and more stable it is. Iodide(I-) it’s a bigger anion than Bromide (Br-) therefore it has more space to stabilise negative charge, and if you can stabilise a negative charge you can make the ion more stable which means a stronger acid. And because Iodide is more stable it doesn’t have a strong affinity for H+, therefore it’s a strong acid, it’ll ionize in H+ and I-
Hope this satisfy your doubt
@@MrChAcHa96 Question what if its HNO3 + H20--->
@@jizla9061 To start you would have to figure out which one is your acid and which one is your base. HNO3 is a strong acid but water has been known to act as either an acid or base. Since you have a strong acid present water would be the base in this situation. Hope that helps!
MY ANGELLL
If I have a option to donate my college fees to you I choose it but in future I want to donate you something for help me in my college education thank whatever your name was literally just thank you sir
OCT is based!
You know why I'm here; Exam tomorrow
What it's something like HNO3 + H20--->
Wdym?
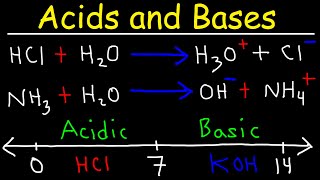
Acid pH value is less than 7 and pH value of base greater than 7
you didn’t explain why HF was the acid and why h2o was the base
He quite literally did
Howd you know we were starting this today lol
badii nem .
Second?
First 😎. Never thought, I would made it. Epic, the best teacher!
First
First 😎. Notification is the real thing! We all in this together!
FACE REVEAL PLS
Need lovely 😍💋 💝💖❤️
I do not like how he explains things.
Said no one ever
first dislike
nice
Noooooooo! Why?
Why?
Worst chanall in the you tube yes na like or dislike