Video není dostupné.
Omlouváme se.
First-order reactions | Kinetics | AP Chemistry | Khan Academy
Vložit
- čas přidán 19. 08. 2024
- Keep going! Check out the next lesson and practice what you’re learning:
www.khanacadem...
The integrated rate law for the first-order reaction A → products is ln[A]_t = -kt + ln[A]_0. Because this equation has the form y = mx + b, a plot of the natural log of [A] as a function of time yields a straight line. The rate constant for the reaction can be determined from the slope of the line, which is equal to -k. View more lessons or practice this subject at www.khanacadem...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacadem...
Volunteer here: www.khanacadem...


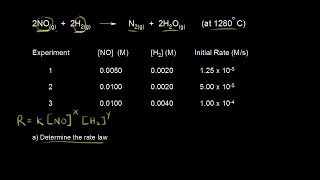






In First order reaction the rate of the reaction is equal ln[A] = -kt + ln[A]. Same as the zero order integrated rate law the slope of the line will be decreasing. With ln[A] vs time.
It is not true to say that the rate law will change as the stoichiometric coefficient changes because the rate law order is only determined from experimental data. We propose reaction and mechanism based on factorial design analysis to postulate the model of rate law. The rate law is nothing to do with the reaction that you write. The stoichiometric coefficient will only be involved in the rate law calculation for other reaction species when the species has different coefficient than the key reactant that the rate law is referring to. e.g. A -> 2B. So the rate law for B is 2(-d[A]/dt)
thank you
nice
I watch this channel for math never science
I like math
I am first 👍
i love it
Wieeee
First view me