Video není dostupné.
Omlouváme se.
Rate law and reaction order | Kinetics | AP Chemistry | Khan Academy
Vložit
- čas přidán 19. 08. 2024
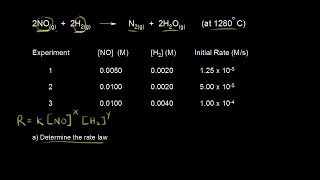
- A rate law shows how the rate of a chemical reaction depends on reactant concentration. For a reaction such as aA → products, the rate law generally has the form rate = k[A]ⁿ, where k is a proportionality constant called the rate constant and n is the order of the reaction with respect to A. The value of n is not related to the reaction stoichiometry and must be determined by experiment. View more lessons or practice this subject at www.khanacadem...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacadem...
Volunteer here: www.khanacadem...







I don’t comment. But this was one of the best videos ive ever watched. So clear and understandable. My Mcat prep course (BP) couldnt even explain it this well. I was stuck and feeling uncertain. This free resource has literally boosted my confidence. Im so grateful right now.
O my allah..!!..thank you for the essential tutorial
Why can't HE be my chemistry professor? I actually get this.
This was REALLY good at explaining the topic clearly and understandably. Thanks!!
I have a Chem final in 3 days and omg this made everything so clear
Thank you 🙌🏽
How did it go?
yes tell us
Yeah dawg, how did your chem final from 4 years ago, go?
@@garchomp3136 Yeah dawg, how did your chem final from 6 years ago, go?
@@nicholasrodriguez2312 dawg how did your Chem final go from 2 weeks ago?
the best explaination ever on chemical kinetics ...Bingo U hit the target of my mind Thanx
thank you! this was a big help
I have A levels chemistry finals tomorrow and you just saved me. *THANK YOU*
How did it go? 😂
Blackisthenewgalaxy two years later lmao
@@naodp I know lmao 😂
jimiiin
this guy teaches me so well
I was about to give up but this video saved me. Thank you so much for explaining this.
Fuck taking chem 2 over the summer.
Im self studying it so I can go into ap Chem. What about you
Chemical engineering. Won't bother to go into my background but I don't want to fall behind such that I can take organic 1 and 2 these next two semesters.
Bro, me too. I'm taking an accelerated 8-week course with one of the toughest professors at my university. Just went over kinetics today.
Nice Gnome child meme btw.
i'm taking chem 2 over the summer now -_-
how did it go? doesn't sound pleasant in the least :/
I was freaking out because my lecture notes didn't have anything on "third order rate laws". Thanks so much!
Two years later and I'm surprised no one has realized that the title is misspelled. It's "kinetics", not knetics.
this video is truly an amazing concept clearing creation of a genius.
I needed Zero order reaction explanation. Thanks, bro.
I think it would be like if you multiplied [A] by 2 but the initial rate would remain the same.
Thank you so much. May God bless him❤
Such a good job explaining this.
Very helpful, thanks.
this guy is SUPERB he reminds me of high school forgoten topics
bless your soul
OMG my lifesaver
Best video for this ever seen
absolute legend! xx
You are awesome! Thank you so much!
Making MCAT studying 10x manageable. Thank you!
Did you sit it yet? What did you get? When i sat it I got 524, I can give general tips if you want
woah that's a crazy score congrats...I want to take it this summer. I'd appreciate any tips you have before I really start going hard on the studying
Clarified it all! THANK YOU
Thank you very much! You saved me from my report tomorrow!
Thankieeeeew so much this was a big help!!
Such a good tutor!!!
excellent explanation
this way so helpful!
thank you
YOU ARE THE BEST
❤❤❤❤😂i finally understand. thank you sir
Thank you so much
THANK YOU!!!!
Thank you so much! Btw you sound like Tyler Oakley
I got a negative and a number in ratio form as my answer eg. -1.765. Any help?
best explication ever Thanks you
Very good lecture
thank you!
Thank you so much , this was very very helpful 💕😩🙏🏾👏🏾
thanks for posting this video.
Great
amazing video. Thank you!!
Thanks very helpful
saved my life
this was a great way to explain this, thanks a lot ! :)
thanku man it was very helpful
Makes sense.
i love this guy
Why there is a difference in the order between different reactants?
Why there is a zero order or a first order .. etc
Thanks
do pseudo first order reactions
thanks
where the hell was this when i needed it in 2013
Is the teacher for organic chemistry Mr. Anderson from Bozeman Science?
eragon2121 no...he's from alagaesia
Anyone know when to use this
average rate of reaction = (-1/a)(deltaA/deltat)
First order and second order
ln(k2/k1) = -Ea/R(1/t2-1/t1)
Hi Garen. For a reactant "a" in a 1st order reaction, { -1/a d[A]/dt = k[A] }. This leads to the 1st order integrated rate law, { [A]_t = [A]_o exp( -a k t ) }. Your second equation comes from the Arrhenius equation { k = A exp( Ea / RT ) }, which describes the temperature dependence of the rate constant. If you plot the natural log of the rate constant versus the inverse of the temperature, you'll find a straight line whose slope is { -Ea / R }, the "activation energy" divided by the gas constant.
The sound is TOO low!
WHERE'S SAL?!?!?!!!!
lmao, i said that out loud too but then i realized it was mean. he did just as good at explaining this as sal would so i'm happy with it
At the end, shouldn't the units for k be mol/L*s ?
obstinate force
kinetics
There's a spelling error in the title. Goddamn it Khanacademy.
Khanh Tran
Khan Academy is two words. Khan...Academy.
Is rate always m/s or it can change?
HELP! what if the one im trying to work out only has 1 table of initial rates? do i do what you did but compare A and B to that one table of initial rates?
You're cute.
what if concentration [A] stays the same and the rate increased by 2 what would happen in that case
What u r talking about is impossible, bcz according to rate law, " (initial) rate of reaction " is dependent on "concentration of reactant", meaning that rate of reaction changes corresponding to change in concentration of reaction. There is no way that u keep the concentration of reactant same , but the rate of reaction changes. If it does, then rate of reaction is not in its initial stage ( meaning "t" will not be equal to 0), but some time will be passed after rate of reaction has started.
6:00 WHAT
im crying
I love u
Thank you T^T
I’m so lost
THANK YOU!!!!!!