Video není dostupné.
Omlouváme se.
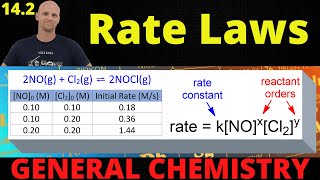
The Rate Law
Komentáře • 265
Další v pořadí
Automatické přehrávání
The Rate ConstantBozeman Science
zhlédnutí 158K
14.2 Rate Laws | General ChemistryChad's Prep
zhlédnutí 108K
Using Gibbs Free EnergyBozeman Science
zhlédnutí 686K
Insane Coffee trick EXPOSED 😱☕️ #shortsWian
zhlédnutí 7M
아이스크림으로 진짜 친구 구별하는법진영민yeongmin
zhlédnutí 12M
【斗罗大陆】坏人居然敢欺负唐舞桐? 斗罗家族可不好惹哟!#斗罗大陆#唐舞桐#唐三#小舞萌萌与舞桐
zhlédnutí 45M
Jak Vypadal Mariánkův Sraz s Fanoušky? #shorts #jonmarianek #marcelMARCEL
zhlédnutí 216K
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical KineticsThe Organic Chemistry Tutor
zhlédnutí 641K
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical KineticsThe Organic Chemistry Tutor
zhlédnutí 1M
Le Chatelier's PrincipleBozeman Science
zhlédnutí 531K
14.5 Integrated Rate Laws | General ChemistryChad's Prep
zhlédnutí 41K
Why is All Life Carbon Based, Not Silicon? Three Startling Reasons!Arvin Ash
zhlédnutí 2,5M
The Most Misunderstood Concept in PhysicsVeritasium
zhlédnutí 14M
Fresh water with 80% energy savings. Revolutionising desalination!Just Have a Think
zhlédnutí 73K
Chemical Kinetics - Initial Rates MethodThe Organic Chemistry Tutor
zhlédnutí 1M
Testing INSANE chemistry recipes from a 1933 formulary book (part 3)styropyro
zhlédnutí 3,7M
FIZI DRINK VS. COCA COLA? Co je zdravější? #shorts #marcel #cocacola #fizistyleMARCEL
zhlédnutí 184K
How I Did The SELF BENDING Spoon 😱🥄 #shortsWian
zhlédnutí 35M
Get 10 Mega Boxes OR 60 Starr Drops!!Brawl Stars
zhlédnutí 15M
How Countries eat spaghettiLionfield
zhlédnutí 13M
IKON A SÉGRA HRAJÍ KÁMEN NŮŽKY PAPÍR CHALLENGE O JÍDLO V BAZÉNĚ ! 😂🍕🍟 #shortsIkonova Videa
zhlédnutí 145K
Harley Quinn's plan for revenge!!!#Harley Quinn #jokerHarley Quinn with the Joker
zhlédnutí 31M
Symmetrical face⁉️🤔 #beautySwasti Ji
zhlédnutí 15M
V MÉ HLAVĚ…Attack
zhlédnutí 570K








I'm a second semester uni student and you are seriously the only thing that allows me to do well sometimes. Thank you x1000 for all of your videos!
so you graduated now ??
Bozeman out there saving lives 🙌
Mr. Anderson,i would like to thak you for making efforts in explaining very complex chemistry , in simple words .thank you ,you are a life saver
who else is here at 5 in the morning before a test?
ten on a sunday morning, my weekends are just this: study study study...and coffee... :)
Conner Pace I got the AP exam tomorrow:/
I’m taking the AP Chem test in 3 hours
Bruh I have it in 30 minutes
@@timmyglasgow4979 How did that go?
The only rate law video that finally breached through my thick skull. Thank you Mr Andersen.
I believe the answer for the 6:50 question Is the rate order is 2. I believe this is correct because the M/s (Molarity/second) concentrations are multiplied by 2. I understand the difference between first-order and second-order as, first order the M/s concentrations are multiplied and the second-order M/s concentrations are squared, cubed, or to a greater power.
I agree
7 years of college and I finally get this concept thanks to this video. Keep up the good work, you're a life (and test score) saver!
Bro help a fellow bro out. Please tell me some sources for physical chem
is the answer: rate = k[A][B]
so a reaction order of 2?
what's the ans?
@@jass2312 it is a second order reaction cuz you add the exponents of A & B and you get 2 cuz each has an exponent of 1
@@Yankee4ever2 wait can u explain how u got the rate for B
@@user-gb2mf7dy5j to get B: keep A the same and find 2 experiements where B doubles and A stays the same (exp 1 & 2): look at the rate, the rate doesnt change, therefore its a 0th order for B hope that makes sense
@@user-gb2mf7dy5jif you include the exponents it would look like: rate = k•[A]^1•[B]^1
Therefore when you add up the exponents it is = 2. (When you raise a number to the 1 power, it does not change. Which is why they don’t include the ^1 in either A or B)
But when you ask did we know that both A and B are first order (^1)? I’m basing my answer off the fact when you look at the chart, when you double the concentration of A you get a Rxn rate of 6, and when you double the concentration of B you get the same answer as you do with A. That informs us that they are equal and likely first order. Had A or B been a second order, the Reaction rate would’ve gone up to 9.
Hey
Hey, thanks for sharing your knowledge to help others learn too
Sup
thank you! helped me review for the ap chem test tomorrow
yo
My man explained clearly in 3 minutes what my professor hasn't been able to clarify in three days. Wow.
I couldn't get it from Atkins' book for days, but you explained it so clearly!! Thank you for your work. You are a talanted teacher.
When you talk about second order reactants at 5:13 and 6:25, I think there is a mistake on the tables. Doubling the concentration of a second order reactant will multiply the rate by FOUR between the trials, not square it. At 5:13, if you work out the rate order for A algebraically (rate1/rate2 = ([reactant]/[reactant])^order of reactant) the math is as follows: 2x10^-3/ 4x10^-3 = (0.1M /0.2M)^x which simplifies to 1/2 = (1/2)^x. X is 1 in this case which would indicate a first order reactant, not a second order one. For it to be second order, the rate for experiment two would have to be 8x10^-3 M/s. Similarly, the rate for experiment 1 should be 32x10^-3 M/s if you doubled the concentration of A again.
There is a similar issue with the example problem at 6:25. The algebraic set up to find the order of A would be 3x10^-3/ 9x10^-3 = (0.1M/ 0.2M)^x which simplifies to 1/3 = (1/2)^x. X would have to equal 2 for it to be a second order reactant, but it does not equal 2. For the table to indicate a second order reactant I believe experiment 3 would have to have a rate of 12x10^-3 M/s. Here is my work: 3x10^-3/ 12x10^-3 = (0.1M/ 0.2M)^x which simplifies to 1/4 = (1/2)^x which simplifies to x=2.
I would love to hear what someone think about this. I am a chemistry student teacher who is relearning kinetics in order to eventually teach it myself so naturally I want to get my facts straight. I very well could be wrong here but in my quest to master kinetics I have found that this video's explanation of second order reactants doesn't jive with other sources I've found such as this resource on page 11:
apchemistrynmsi.wikispaces.com/file/view/12+Kinetics.pdf
I just went over this with my students, who watched this video for homework. You are right. I think that Paul was thinking that the rate would be squared (e.g., 3^2 = 9), which is where he got the 9 in the table instead of 12. But the rate should increase by a factor of four, like you are saying.
Dauson Larrabee great job wow I think you are right
Dauson Larrabee and thank you
Agree, there is a mistake in the video.
You need a Nobel prize.. You are out here saving lives!
That because some of us are fools studying right before test or just have teacher who can't teach
chemistry is hard for people though. So we use this to understand more.
Nobel Prize for Peace
After I finish the AP Chemistry series I will start on an AP Physics series.
🍎Oh Soo fun! Like sugar before bed.🤣
This is SO timeless, thank you SO much for helping out students even after 10 years!!
Thank you. You explained it better than my textbook did.
studying for the MCAT and this super helpful! Thank you!
The overall reaction order is 2, no?
1 and 1, right?
@@ahmedelsaid173 yes I think so too
Two questions:
1. In the context of reaction orders for a reaction involving multiple reactants, how does one determine the order with respect to each individual reactant and subsequently calculate the overall reaction order?
2. Explaining rate constants (K) to someone unfamiliar with chemistry: How can you elucidate the significance of rate constants in the context of the rate law and their role in quantifying reaction rates?
For the question at 6:50, i got rate=k[A][B] (I am assuming I don't need to put the 1's since it is already implying first order for both). Correct me if I am wrong please.
Also, that video was very helpful.
+MoeBro25 yes that is correct
It its correct but overall its a 2nd order reaction
Incorrect. It should be rate = k[B] because A is zero order. When you doubled [A] the rate stayed the same, meaning it would be to the 0 power.
I think the rate of each experiment is considered overall for that experiment. In experiment 1 they both started out with 100 M and that resulted in a rate of 3 *10^-3. In the second experiment the change was made to reactant B where it M was increased to 200 (reactant A remained at 100 M), then the "overall" rate of that experiment is 6*10^-3( first order because it went from 3 to 6). And in experiment 3, a change was made to reactant A but not B (I'm assuming that we are making our basis of changes around the standard 100 M of a reactant, so be is at 100). Similarly, the increase in M of reactant A in experiment 3 causes the "overall" rate of to be 6*10^-3 (first order because it went from 3 to 6 [compare experiment 1 and 3]). According to this video, the "overall" rate of reaction for this REACTION (not saying experiment here, we concluded this based on the EXPERIMENT though) would be to add the two order (exponential properties here?) of the reactants together. 1 + 1 = 2 or 2nd order.
I believe the difference between zeroth-order, first-order, and second-order are the rate/time graphs that each create. The first-order is a constant rate over time (straight horizontal line). The second-order is a straight line with a decreasing slope. The second-order is a curved decrease of rate over time.
Wishing you were my college chemistry teacher the way you teach things so clearly and concisely
I have two questions:
1. Considering the information on reaction orders discussed in the video, how would you determine the overall reaction order for a reaction involving multiple reactants?
2. After discussing the rate law and reaction orders in the video, how would you explain the concept of rate constants (K) to someone new to chemistry?
Bozeman’s the real MVP. I actually feel like I might pass the AP exam tomorrow 😊
did u pass lmfao
I think the answer at 8:40 min should've been R=K[A]
Since they both double which means they are proportional and that says it should be the first order NOT the 2nd
Thank you so much Mr. Anderson, this have made my life soooo much easier!!!
this was so helpful, my exam is tomorrow and I feel a lot better already! THANK YOU
You explain things so well and with quality. Thank you for the solid explanations
Steve Kerr if he didn't hoop.
Joshua Ramirez lmao
Paul, please fix your mistakes for the two second order examples. If you double the concentration for a reactant that is second order, the rate will go up 4 fold, not the square of the original rate. Your stuff is so well done, I love to use your videos, but I cannot use this one with that mistake.
Thank you- I was searching in the comments to see if I was incorrect in noticing this also, or if anyone else had pointed this out!
Hello, Mr. Anderson. Thanks for the tips.
Nice explanation and easy tricks to solve problems. Thank you :)
Thanks a lot. You teach well and concisely. Students will be benefited from you. Keep up!
How does the fluidity of the cell membrane change in response to changes in temperature?
At 6:28 if you solve that algebriacly you get approx. 1.58
(9*10^-3)/(3*10^-3) = k*0.2^m*0.1/(k*0.1^n*0.1)
3 = 2^m
m = log_2(3)
Very good and simple explanation of the topic highlighting how we can identify each order.
If I may, can you elaborate on any limitations or exceptions where graphing might not be the best method to determine these orders?
you da man mr A
At the min 3:28, the decomposition of ammonia was discussed in terms of reaction order. If the concentration of ammonia does not affect the rate; does this imply that temperature and catalyst presence are the only factors that can change the rate of a zeroth-order reaction?
So, [A] is 1 order and [B] is 1 order. Is the overall rate is 2?
+Luph9113 yes
If the order of [B] would have been 2, then the overall order of the reaction would have been 3. Third order reactions are generally the highest order reaction possible because higher orders require more molecules to react at the same time. Hope that helps!
Most Chem teachers don’t go beyond 2nd order for the sake of the student and the overall applicability of the concept, but there are orders beyond 2nd order
@user-up8se8yf7s
Yes, a reaction can have a zero order with respect to one reactant while having a non-zero order with respect to another reactant. This situation arises in complex reaction mechanisms where the reaction rate is determined by the slowest step, which may involve different reactants.
this guy just saved my midterm !!!!!!!
Amazing job!!! Went into Chem lecture confident, left confused as fuck!! But after watching this simplified version the basics help make total sense of whats to come. Thank you bro!!!
thankyou for this nice explanation and example which you took has made me to realize the concept well
When we compare first-order and zero-order reaction graphics for the disappearance of reactant A with time, at which times during the reaction would you have trouble distinguishing a zero-order reaction from a first-order reaction? Thanks for your awesome tutorials. You explain every detail in an easy way to digest. This is the way! :)
When time is closed to zero.
To distinguish between zero-order and first-order kinetics, you need to conduct experiments and analyze how the concentration changes with time. Zero-order reactions show a linear decrease in concentration, while first-order reactions display exponential decay. So, conducting experiments and examining the reaction rate with respect to concentration is the most effective way to determine the reaction order.
Thank u for Your Gr8 work Mr Anderson !
I love your videos..these r really helpful to even a guy like me who's sittin here in India..!
i hope someday i'll be able to meet u and express my gratitude to u in person..!
7:26 How do you calculate which order it is if after making a spreadsheet and filling the data, none of the graphs are a straight line?
Doesn't a second order straight line have a positive slope? 1/[A] = kt + 1/[A]( from integrated rate law) where 1/[A] is on the Y axis and a a slope = +k?
I also think that second order would have a positive slope.
Thank god you did this , exam in two weeks
just amazing explanation thank you
phenomenal. please continue making videos you are an excellent teacher
I understood that the decomposition of ammonia is a zeroth-order reaction because the rate remains constant regardless of concentration changes. But how do we determine the reaction order for more complex reactions with multiple reactants?
thanks alot Mr Andrewson :***
*Anderson
I really like how you explain everything, but I still have a little confuse on the overall reaction order. Can you explain the part were you identified if it is a zeroth-order, first-order and second-order? I would appreciated very much.
God bless you!
This was incredibly helpful. Thank you so much!
How does changing the concentration of reactants in a chemical reaction impact the determination of the rate law order, and what role do experimental methods play in determining the order of reaction?
answer 6:48
order of A=1
order of B=1
K=0.3
rate=3x10^-3
Outstanding. Bravo.
How is the rate law for a zero-order reaction written? What are the units of the rate constant for a zero-order reaction?
That was very helpful! Thank you!
This helped me understand my lab, we did the crystal violent thing I have no idea what we did I did my lab report with general things like when x=0 and 1 it'll be decreasing, and only when x=2 it'll increase, for the second trial I got a bit of a curve and I put that I there must have been inaccuracies when I did the lab, not entirely sure what it meant but I do understand it a bit better after the video I'll re watch this when editing my lab since it's not due for another 2 days
This video is really helpful 🙌🙌 Thank you so much 🌟🙌
Thanks!
When one has more than two reactions orders, how does one establish who goes first in the rate order, how does one add up both rates and how does one establish the graph of two reactions?
This video was extremely helpful however when looking at the table of data shown at 6:08 you said that for compound A we double the concentration but because the rate is proceeding as a square the overall order must be 2. but isn't 3 multiplied by 10 to the -3 squared , 9 multiplied by 10 to the -6 instead of 9 multiplied by 10 to the -3?
Considering the information about reaction orders discussed in the video, how would you determine the reaction order for a reaction involving multiple reactants?
Thank you! I have a question:
We know that in a zeroth-order reaction, we can change the concentration, and the rate stays the same, but what happens if we change the temperature?
Absolutely amazing! thank you so much, this was so helpful :)
a=2nd b=0, overall reaction rate is 2nd (2+0=2)
+Luisa Rodriguez i guess both of them were first order and overall rate it 2
+Luisa Rodriguez Both of them are first order, so overall is 2
Superb video ❤😊
You are awesome. Thanks.
Does the atmospheric pressure influence the rate of reaction? If so, how do the changes in atmospheric pressure influence the different orders of reaction?
Is the a physics series ? Your videos are always awesome and very informative! Keep up the great work :-D
Bro it's chemistry 🗿
How does the rate of reaction change over time for a zeroth-order reaction compared to a first and second-order reaction, as described in the integrated rate laws for each reaction order?
This man is the MVP
Hello Mr. Andersen, at 6:21 : isn't the rate proportional to [A], so if the rate is increased by a factor of 3 (between the two trials), then [A] doesn't make a sense that it's doubling, right?. I am trying to match it with what I have in my textbook but I don't think i understand
How does the half-life of a reaction change with concentration for zeroth-order, first-order, and second-order reactions?
what are the key steps involved in determining the rate constant and overall reaction order from experimental data?
So helpful! Thank you!
Thanks
Would the correct answer for the table at 7:00min be first order reaction (rate=k[A])?
This was helpful. Thanks a lot
Thank you.
thank you
4:38 shouldn’t it the rate over time be exponential too since rate is proportional to the concentration of A. Wouldn’t the graph of the rate over concentration of A make the straight like instead?
I think the straight slope for the 2nd order reactions must be increasing with time since it inverse of the concentration
Thanks a lot much respect
Isn't the reaction to the "zeroth" power suppose to be a straight line because (k) is a constant for that specific reaction and the first order is the one that makes a diagonal line
?
never mind its the [] to the time graph not a rate to time graph my bad xd
you're a genius
I have doubt about a specific section, would a reaction whose overall rate is second-order behave similarly to a simple second-order reaction?
Excellent video, I have a question.
How do changes in temperature and concentration of reactants influence the rate of a reaction? And with respect to the spectrophotometric analysis, is the absorbance equal to what the concentration has been? thank you
You're awesome, thank you!
rate = k[A][B] ??
Mr. Anderson I would like to know the answer.
Thank you for your great videos!
That just means there are two reactants. Like reactant A + reactant B (HCl + NaOH > ...)
I believe he is correct, Megan. He is giving the overall order, not a chemical equation. Because B is constant in Samples 1 and 3, we can use them to Isolate A. Because both the rate and concentration of [A] in the 3rd is exactly half at half of their respective values from sample 1, we know that m (order of [A]) is 1. Using the same technique for [B] by isolating Samples 1 and 2, which concentrations of [A] are static, we also get an order of 1 for [B].
Thus, R = k[A][B]
Roy O'Neal I thought he was asking what the notation meant. I didn't know he was answering a question..
How do we determine that graphing the inverse of concentration over time results in a straight line connected to k without requiring advanced calculus, as discussed from 5:40 to 5:45 min? Is there a specific concept or method that support this?
this is great! thank you
If one were to be utilizing a method of collecting the data of a reaction, like spectroscopy for example, could the data collected of the emission or absorption be thus put in place of the concentration in the table and graph from there?
Great vid, thanks
Thank you that was really helpful :D
i don't get how the second order is faster based on the graph
Thanks! this is very helpful :D