Exceptions to the Octet Rule
Vložit
- čas přidán 11. 07. 2024
- There are many exceptions to the octet rule that you should be aware of in chemistry. In this video we go through a list of exceptions with an explanation for each and examples.
Knowledge of these octet exceptions is important for drawing Lewis Structures. When drawing Lewis dot Structures that involve elements which may be exceptions to the octet rule, you can calculate the formal charges to be sure you have the best/most likely Lewis Structure for the compound.
----Lewis Resources----
• Lewis Structures Made Simple: • How to Draw Lewis Stru...
• More practice: • Lewis Dot Structure Pr...
• Counting Valence Electrons: • Finding the Number of ...
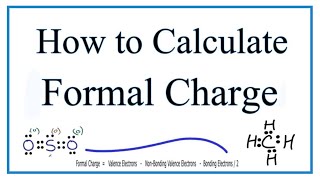
• Calculating Formal Charge: • Formal Charges: Calcul...
• Exceptions to the Octet Rule: • Exceptions to the Octe...
--Steps to Write Lewis Structure--
1. Find the total valence electrons for the molecule.
2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside.
3. Put two electrons between atoms to form a chemical bond.
4. Complete octets on outside atoms.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
Lewis Structures are important to learn because they help us understand how atoms and electrons are arranged in a molecule. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule (like boiling point and surface tension).
Chemistry help at www.Breslyn.org







What is most confusing exception to the Octet Rule for you?
--- Dr. B
Wayne Breslyn
For me its sulphur nd phosphorus..... I cant understand them at all... That was a nice video... Thanks for being helpful😍😍
Does having an expanded Octect affect how the formal charge of the central atom is calculated?
Does it have any order to put dot
Sulphur
Wayne Breslyn
Boron and Aluminium are confusing
Wayne, your videos have been extremely helpful during this stressful semester. Thank you so much for your diligence and wonderfully simplistic style, you make every concept so much easier to understand.
Glad I could help and thanks for the kind words!
4:02
NO (yes!)
Lmao 😂😂😂
You are youtube
These are gold, man!
Thanks! --- Dr. B
Oh gosh! Finally I have someone for the chemistry.
Excellent!
Really thanks sir
2:23 Expanded Octets
Thank u very much for making this helpful video!!!
No problem, glad I could help with exceptions to the octet rule! --- Dr. B
Sir what is the relation between Lewis structure and octet rule and tell me please that the compounds which are exceptions for octet rule also exception for Lewis dot structure rule
Sir is their any more exception or only these are all the examples who don't follow octate rule or is their infinity number of exception in octate rule ? Can you tell me sir
Oh ! Its amazing
Thanks, such an amazing explanation.
I like the way you teach & the way you teach is clear & easy to understanding but i can't get some topics in my grade 11 chemistry
thank you so much!
You're welcome!
amazing it was just like magic
Excellent!
How is the Lithium cation formed, did it have to undergo any chemical bonding to become Li+ or did it just randomly lose an electron to complete its 'octet'? Or is this just the result when Lithium reacts with a non-metal to form an ionic compound, meaning you only singled out what would happen to lithium in a reaction technically?
Thank you so much this is so helpful!
Thank you! Dr.B
No problem! --- Dr. B
Nice video, greetings from Paraguay
Thanks!
I've never been to Paraguay. Perhaps one day... -
-- Dr. B
Thanks!
Thank you very much, it is appreciated! (I just learned how to filter my comments for people who gave Super Thanks. )
Thanks 👍
Just so you know, someone did notice that easter egg at 4:03.
*YES!*
Thanks Teacher
Welcome!!
SCl4 has an expanded octet right?
Sorry, could pls tell me which atoms make expanded octets?
Excellent
Thanks!
H, Li and Be it's not exceptions from noble gas rule (dublet and octet). But boron is! Stable configurations is 2 and 8 electrons in the outer shell. Another stable configurations in group 3-12 of periodic table is half filled and fulfilled orbital d (d5 and d10).
Thank you very much for this video. However I have a few questions:
1. Boron and Aluminum only require 6 valence electrons to be "satisified" in structure. However, if required could Boron and Aluminum each be able to fit/accept/share an additional 2 valence electrons, or is 6 electrons the maxium either of those will accept?
2. What elements does the "expanded octet" rule apply to? I think you said in some other video that this rule applies to elements after aluminum (silicon onwards).
2. Mostly the elements in the 3rd period cuz they have empty d orbitals and u can form PCl5 and then SF6 or SF4 or NCl5 OF6 (CF6)-²
What about beryllium, doesn't it only need 4 Valence electrons?
Thank you very much for making this helpful video
Glad I could help with those exceptions! You might even say the video is "exceptional"...
--- Dr. B
@@wbreslyn 😂😂
@@DrAdityaReddy 😎
Shouldnt sulpher have 18 valence electrons to complete the 3rd shell? Why just 10? I am confused
How does formal charge help determine if the Lewis Structure is the best? Is it basically if the formal charge is zero, then that is the best one?
That's the idea, the closer to zero the more likely the Lewis Structure represents the molecule in the real world. Here's a video I did on formal charge that might be helpful:
czcams.com/video/-9f4H0puVzc/video.html
--- Dr. B
is very nice thank you very much and good job profesar
Thank you! --- Dr. B
*professor
Beside for noble gas, Octet rule is majorly for Neon-near elements (Carbon, Nitrogen, Oxygen, Fluorine, Sodium, Magnesium and Aluminium)
Awesome
Thanks!
Which compound will be the most stable the one with the least formal charge on the one with the most formal charge
The one with atoms having formal charges closest to zero.
Take a look at these videos:
Determining Formal Charge: czcams.com/video/vOFAPlq4y_k/video.html
Formal Charge Practice Video: czcams.com/video/-9f4H0puVzc/video.html
--- Dr. B
Hey, thank you for making this video!
I have some doubts though ~
∆ *why* can some elements have expanded octets?? Is the only explanation that "the central atom uses its d orbital?"
∆ If yes, why can't *any* element use its "d" orbital when its in the center and thus have an expanded octet?
∆ I even saw a Lewis structure with Xe having 14 electrons around it. What is the upper *limit* on what an atom can have around it in a Lewis structure?
I'd be really grateful to anyone who can help, this concept of expanded octets is really bugging me..😥
Take a look at this page (it's a way down) where they explain about the d orbitals. Note that it isn't until Period Three that d orbitals are used by atoms.
chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding/Violations_of_the_Octet_Rule
--- Dr. B
Wayne Breslyn
Thank you for replying, first off! I just read the article, Dr B, but I still don't get it.. Please could you just write like a 1 sentence answer each for the 3 questions I have? That way you don't have to waste much time explaining! I'm sorry for troubling you!🙏
blissful fragrance
The answer to your first question is that yes, electrons fill the d orbitals of the central atom. This is only relevant to atoms in period 3 and onwards due to thes atoms actually having the d orbitals available due the the energy levels that the electrons are found in. For example, Carbons electron configuration is 1s2, 2s2, 2p2 and has 4 electrons in the valence energy level/electron shell. Carbon therefore needs 4 more electrons to fill the valence shell, which is how CH4 is made :D
If we were to try and expand the octet rule.. adding more electrons would ultimately start filling up the 3s and 3p orbitals, making the electrons in these orbitals the valence electrons rather than adding to the 2nd energy level of the atom. This is because energy level 2 only has one s and one p orbital. Electrons cant just fill the 3d orbital due to the fact that electrons will start filling the next energy level making the 2nd energy level electrons no longer valence electrons... if that makes sense? Atoms in period 1 and 2 cant fill up the d orbital without filling up the previius orbitals (3s 3p and 4s)... so a lot of electrons are going to have to be added to fill the d orbital, which is why group 1 and 2 cant expand the octet rule.
The larger molecules are a little more complicated ...
Xe has an electron configuration has of [Kr] 4d10 5s2 5p6 and has 8 valence electrons. Xe can expand due to the fact that it has a 5d and 5f orbital.
However im unsure if the electrons will try to fill the 4f orbital or if it will just fill the 5d orbital due to the fact thar we are twlking about the octet rule and lewis structures rather than the electron configuration itself... I hope some of this is helpful 😂 chem gets complicater as heck
I have a question that has been baffling me since high school.
The octet rule says that atoms tend to gain or lose electrons in order to achieve a "noble gas configuration," which for main group elements would be 8 electrons in the valence shell. For transition metals, for example, when they are bound with ligands, they tend to go for 18 electrons in the valence shell.
So my question is, what is a "valence shell"? Is it an energy level, like KLMN or 1234, or is it a single sub-shell, like an spdf? For example, would it be correct to say "the valence shell for an Iron atom would be 3p, 4s, and 3d, as they are the highest energy 18 electrons"? Or should I say "the valence shell for an Iron atom would be 4p, because iron achieves a noble gas state by filling it from ligands"? Thanks for any help with this!!!
It's a outer most shell...
the valence shell is the last shell in an atom. For example O: it is 8 soit has 6 electrons in its last shell.. so the valence shell of Oxygen is 6.
gracias viejito
De nada, pero no soy muy veijo!
Thanks mayne
No problem, glad I could help with exceptions to the octet rule!
Thanks dr B
Always welcome!
Good
is there any way to predict the limit of expanded octets? your videos are great but you didnt really explain why expanded octets have the properties they have: to what extent do the D orbitals hybridize?
Actually I’m not sure on that one. Fourteen seems to be the limit, though. Take a look at this discussion…
chemistry.stackexchange.com/questions/27998/what-is-the-highest-possible-expanded-octet
This video is a more introductory treatment to the exceptions, so I didn’t go too far into the interesting concepts you mentioned.
--- Dr. B
I don’t really understand the Boron and hydrogen example?
How to check best Lewis structure using formal charge?
Like this:
Determining Formal Charge: czcams.com/video/vOFAPlq4y_k/video.html
Formal Charge Practice Video: czcams.com/video/-9f4H0puVzc/video.html
--- Dr. B
But Al is not exception he nees to give 3 electrons(opposite of N)
You mentioned that expanded octets occur on the third period from Silicon and below. P is on the same period as Si, and so is Cl. On the PCl5 example, Cl is considered complete with its outermost shell filled with just 8 valence electrons, completing the octet. My question is, what if Cl happens to be the element on the center of the lewis structure? Will it be able to carry more valence electrons than 8 or it’s limited to 8?
Take a look at this video (see how Cl isn't limited to 8):
czcams.com/video/bXEMU2fMMus/video.html
--- Dr. B
👏👏
Is water an exception of the octet rule?
Only that H atoms only need two valence electrons to have a full other shell.
what are formal charges? and how do they help to check for the most likely Lewis structure?
Here you go!
Determining Formal Charge: czcams.com/video/vOFAPlq4y_k/video.html
Formal Charge Practice Video: czcams.com/video/-9f4H0puVzc/video.html
@@wbreslyn wow thanks so much! your videos really help me with chemistry we learn in school
God bless you.
Thanks! --- Dr. B
why didnt u use line??
Thanks for this nice video to understand this exceptions. Even as a German it was easy to understand.
Glad it was helpful!
How to find out that an atom follow octet rule or not if only an atom is given in the question like O, P, C? Plz answer plz plz
You can't really. You've got to write the Lewis Structure and look at the formal charges. --- Dr. B
How to Draw Lewis Structures: czcams.com/video/1ZlnzyHahvo/video.html
Lewis Structures Practice Video Worksheet: czcams.com/video/DQclmBeIKTc/video.html
Determining Formal Charge: czcams.com/video/vOFAPlq4y_k/video.html
Formal Charge Practice Video: czcams.com/video/-9f4H0puVzc/video.html
4:04 yes!yes!yes!
At 3:10 how can you use so many electrons if phosphorus has 5 valence electrons?
Actually I got it now, thank you
Excellent! --- Dr. B
In metallic bonds, do metals follow the octet rule?
Good question! The folks at Socratic did a good job explaining:
socratic.org/questions/how-does-the-octet-rule-affect-metals
i have a question
in the case of ionic bonds, like the one bw sodium and chlorine, can we represent it as a lewis dot structure/
Yes, but we need to be clear that electrons aren't shared in the bond. Like this:
czcams.com/video/nQyOaEtboC8/video.html
How do we calculate the formal charge Dr.B?
Formal charge= Total number of valence electrons in the free atom - total no. of lone pairs electrons - 1/2xtotal no. of bonding electrons.
why does lithium lose the valence electron?
Since it's only got one electron in it's outer shell it will lose that electron. That way the shell goes away leaving a full shell underneath. In the case of Lithium that would mean the first energy level/shell which only needs two electrons to be full. In a larger sense Li loses the electron to form a chemical bond and lower its energy and become more stable. --- Dr. B
What about transition metals.Let's say Fe^2+. Iron then has 14 valence electrons.
In general we don't draw Lewis Structures for transition metals. It gets messy very quickly...
--- Dr. B
@@wbreslyn Thanks.
For me BeCl2,BCl3
Here you go ...
czcams.com/video/N4jhHNndHp8/video.html
czcams.com/video/8l1IoCVWtI4/video.html
--- Dr. B
Which of the following species does not follow octet rule and not act as Lewis acid-(1)pcl5 (2)CO2 (3) H- (4)SO3
What about Fe 2+ and Fe 3+?
Also, why does the O get all the electrons (4:38), and not N? Why couldn't it be the other way around?
O has a higher electronegativity so it might attract the electrons if am not wrong...
Phosphorous is also confusing
Agreed. It can have an expanded octet. --- Dr B
NO
Yᴇs!
me: says one word to any girl
the girl: 4:05
We've all been there!
you couldnt just put them in a list, how the fuck does this video have 131k views
How is the Lithium cation formed, did it have to undergo any chemical bonding to become Li+ or did it just randomly lose an electron to complete its 'octet'? Or is this just the result when Lithium reacts with a non-metal to form an ionic compound, meaning you only singled out what would happen to lithium in a reaction technically?