Formal Charges: Calculating Formal Charge
Vložit
- čas přidán 16. 05. 2013
- A step-by-step description on how to calculate formal charges. Formal charges are important because they allow us to predict which Lewis structure is the most likely to exist in the real world.
Get more chemistry help at www.Breslyn.org.
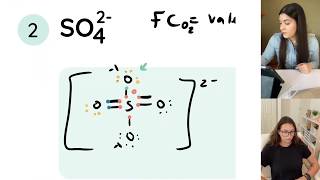
Often you are given a compound with more than one possible Lewis structure. Often this is the case with elements like Sulfur or Phosphorus which can have more than eight valence electrons. In these cases it is important to calculate formal charges to determine which structure is the best. The structure with formal charges closest to zero will be the best.








Thank you so much.. my professor was killing me with this.. but you made it so understandable
You are a beautiful teacher. Best ten minutes i have spent online today cause i watched it twice.
Dr. B, This is so simple..... Thanks a lot. You're a life saver.....
Outstanding job explaining this in such a simple way. Keep up the great work Dr.B!
out of the million videos, this one helped me the most thank you.
One of the best chemistry professor i have ever witnessed
Thank you Dr. B. Your videos helped me realized that I'm capable of learning chemistry.
Thank you so much Dr.B .Amazingly explained and a very useful technique in chemistry. I wished I would have learnt about this in high school, it would have made structures so much easier to draw. I wish your channel grows much more so more people can benefit from such knowledge and wish you good-luck with it.
Awesome lecture. I understood very well. I didn't saw this type of lectures upto now.
My professor literally overcomplicates everything, and this made my life a lil bit easier tysm.
you are Better than anyone who has ever tried explaining this stuff to me.Thanks
Thank you so much, Dr. B! You're so good at explaining chemistry! You're the Bruce WAYNE of chem!
You are saving me from my online professor that BARELY teaches, THANK YOU SO MUCHH
Woah... I actually understood. If only I get the Chem Lectures just as fast I learn in YT. Thanks!
thank you soon much , if this will come to me in the exam i'll remember your video. it's so easy thank you again
Thank you for this video.
Thx man, you're helping me in final exam :D
Thank you so much. This video will really help me. Our teacher just gives us answer sheets without explaining anything and yesterday she just made us answer Lewis Structures lol. Thanks a lot!
Thank you so much, I have a test tomorrow and I just couldn't wrap my head around this concept. Your video really made me understand it!
Simple and helpful- thanks for the video!