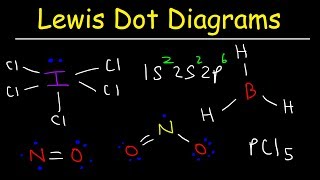
Exceptions to the Octet Rule - AP Chem Unit 2, Topic 5B
Vložit
- čas přidán 27. 08. 2023
- In this video, Mr. Krug works through several examples of molecules that are exceptions to the octet rule and exhibit and expanded octet. He shows students how to recognize a molecule that will be an exception to the octet rule.








A much more understandable explanation, thank you.
I'm so glad you found it helpful. Thanks for watching!
Does that mean that any central atom can have an expanded octet, even if it is in the second period (below the third period, as the exception stated)? Or is it still just for the third?
Expanded octets are reserved for atoms in the 3rd period and below, since they have d orbitals and can use those for hybridization.
i love u
Thanks!