The Octet Rule: Help, Definition, and Exceptions
Vložit
- čas přidán 25. 11. 2017
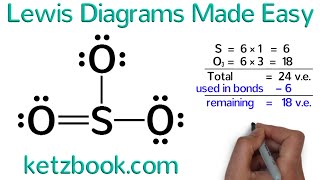
- The Octet Rule is a general rule that is used to describe chemical bonding and draw Lewis Structures. The rule states that Main Group elements form bonds in a manner that results in each atom having eight valence electrons in the highest energy level (sometimes called outer shell). This results in each atom having the same electronic configuration as a noble gas.
For a complete tutorial drawing Lewis Structures, watch my video:
• How to Draw Lewis Stru...
The Octet Rule doesn’t work all the time and there are many exceptions. Hydrogen is one of the most notable exceptions and only needs two electrons to fill its outer shell. There are numerous other exceptions including expanded octets which can have up to twelve valence electrons.
Even with the exceptions, the Octet Rule is a valuable rule of thumb that helps scientists predict how atoms will bond to form compounds. These predictions are often what is ultimately unobserved in the lab.
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).








his voice told me that everything was gonna be okay
This comment made me smile!
especially for my chem test tom
Yeah , without my headphones
when some one notice me.
I am so proud for his voice
@@wbreslyn which software you use for video
@@poojaboutique9147 Good question. I use a number of tools and increasingly I’m trying to use only open source software.
Lately I’ve used InkScape (inkscape.org) for the writing. It is vector based so it gives the writing a very smooth feel. Adobe Illustrator was a bit expensive and I wanted to use software that was available to everyone.
For image manipulation I use GIMP (www.gimp.org).
Animations are usually done in OpenToonz (opentoonz.github.io) although I’ve not done much lately since it takes a lot of time.
Often for molecular geometry I use phet.colorado.edu/sims/html/molecule-shapes/latest/molecule-shapes_en.html.
MolView: molview.org/ is really helpful as well.
For screen recording I use Camtasia Studio (costs money). This isn’t open source. I also do my cleanup and editing using Camtasia Studio. Many of the fades and transitions are done in Camtasia. OBS Studio (obsproject.com) is a free capture program that I’ve been meaning to try.
I’m using a Dell Inspiron notebook computer with Windows 10 and a Yeti Blue Microphone. My writing tablet is a Wacom Bamboo.
There are a lot of moving pieces, but they make the process more enjoyable and the outcome more useful.
This whole week I've been struggling with Lewis dot and octet rule. I literally learned how to do both in less than 5 minutes from this video and another video. My professor is so trash. You are not trash sir thanks for the help
Glad I could help! --- Dr. B
"You are not trash sir" that's an interesting way to compliment someone. lol
@@irisce2799 LOL frr
@@irisce2799I am dying, with so much stress thank your posting this comment I appreciate you
This channel helps me understand more than my science teacher.
Thanks! --- Dr. B
Honestly if your teacher can't explain the octet rule you're kinda fcked
@@ironwithin3875 lol I finished my ap chem and got my diploma in without knowing the octet rule
Getting ready for finals and this taught me 3x more about the Octet rule 😂
um same... oof
I survived 7th and 8th grade without knowing the octet rule, it's time.
Damn hahahahah
same
I'm learning it now in 11th grade 😂😂
@@ZD-vz1hw Same, literally have a test in like 40 minutes
Thanks, now I know where to go when my teacher becomes Cardi B in explaining the topic
ur the only youtuber that actually helps me understand. i think its the tone of ur voice being so clear and direct, as well as ur simple realistic explanations that doesn’t overcomplicate things. ive got a whole unit test tomorrow and never had the best grades for chemistry this year because of my teacher over complicating everything
Your videos are more than appreciated, Sir! Thank you for helping this old lady get through chemistry, Dr. B. 🥰
My pleasure and all the best in chem!
Thanks, that was simple and straight to the point! Gave you a like to support the channel. Keep up the great work!
Awesome, much appreciated! --- Dr. B
U git no clue hiw many students will thsnk for theur life for providing these vids 🙏🏻
Such a helpful video. Thanks for everything you do
Glad I could help with the Octet Rule! --- Dr. B
i honestly prefer short, direct and simplified videos like these ones
So do I!
I have been trying to understand this. Thank you for this video.
Happy to help!
LOVE Dr Bresyln's Videos. Thank you for taking the time to share your knowledge and for making this easy to understand:)
Your video help me lot in chemistry
Excellent, glad I could help! --- Dr. B
Thankyou Sir I now have the answer to my doubt in spit of the fact that i had to watch 4:30 minutes of some content that wasnt my question but knowledgable. It was just weirdly helpful to watch as you gave my answer in the last 10 seconds.
Good work, keep it up!(im saying geuinely)
My teacher was yapping for almost half an hour when teaching this same thing, yet you did it in only 4 minutes. thank you.
Dude thank you Dr B for expalining slowly and not overwhelming me like my teacher does
No problem, glad I could help with the Octet Rule! --- Dr. B
Thank you! For some reason I couldn’t get this for so long, this cleared things up💛🧡❤️
Simple and short, thank you very much sir!
You are welcome!
Thankyou soo much instead of 20min lecture you taught us in 4:45min soo helpful video
I've subscribed your channel
Excellent!
Thank i sooooo much. I was struggling immensely with this. Thank u sir
Thanks for telling nicely this video made doubts clear
well taught man, love ur voice.
Really its awesome and easy understanding
Who are those 88 idiots who disliked this wonderful explanation. ....
Sir. ..you explain a thousand times better than my science teacher ...I don't mean to hate my teacher but ....You're great ....from now onwards I have a respect for you in my heart
Thanks for the kind words! They are appreciated.
well explained sir
Thank you so much sir! I understood everything 👍🏻
Glad it helped!
Very good explanation 👍🏻👍🏻
It helped me a lot in understanding this concept.
Thankyou 😊
Most welcome 😊
Helped me so much for my test tomorrow lol, thank you
Oh man you are really a cool teacher and thanks for explaining this topic .
Glad it was helpful!
Thank you i have clear all my basic concept
Excellent, the octet rule is important in general chemistry. --- Dr. B
Great video thanks a lot
Glad I could help with the Octet Rule! --- Dr. B
Your voice is so calming
Thank you!
Thank you very much!
I genuinely thank you
You are most welcome!
Thanks for guide
thank you for this!! i'm struggling on that octet rule but you explained it neatly! thank you!! ♥️
You are a LEGEND FOR REALLLL
Thanks! --- Dr. B
I dont know how i made it to the english science part of youtube as a polish person, but this is way better than polish videos.
I have a question, I know the way your predict how many electrons fall into each layer (2n2) but how do you do that with atoms that have an odd number of electrons? I notice it doesn't work quite as well. I.e., Unbiunium. It's shell structure is 2,8,18,32,33,18,8,2. Any pointers?
This is very helpful thank you
You are welcome! Glad I could help with the Octet Rule. --- Dr. B
Thank you so much it helped em alot 😊
Thank you man now i get it
You deserve more❤️🌸
I understand very easily great
This was Sooo useful😭😭
Crystal ✨ clear explanation
Sir you are really legend in chemistry
what a nice explanation,thank you
Thank you - most appreciated! -- Dr. B
Thank you :D
You are most welcome!
I love your videos dude Keep it up
Thanks, will do!
It helped me a lot in understanding..the octet rule
Excellent --- Dr. B
Thanks dude, that really helped
Awesome, glad I could help with the octet rule! --- Dr. B
@@wbreslyn really loved all your chemistry related videos and your website was a great help! 😄
Thanks for the encouraging words! I'm still working on adding content to the website.
Thanks!
Crystal clear definition👍👍
Thank you, I spent a lot of time creating this video! --- Dr. B
@@wbreslyn Sir can u try to upload a video about dipole moment...... It would be of great help if u do so..
This might help some...
Polar/NonPolar: czcams.com/video/OHFGXfWB_r4/video.html
Thank you
Thank u for a such a nice video
My pleasure 😊
my chemistry grade was so low with this video i think i will pass before the regents exams
I hope you pass!
Thank U Sir...
This helped me toooooooooooooo much,Thank You
You're welcome!
thank u dr. b sir
Thank you sir❤
You are most welcome!
Sir, can I take down notes of what you said in your video and share it to my classmates? I will make sure to put citations. Thankyou
Thanks for this nice video
You are welcome! --- Dr. B
Thanks a lot!
No problem, glad I could help with the Octet Rule! --- Dr. B
thank you so much !!
You're welcome!
Nice explanation....
Thanks! --- Dr. B
How does the octet rule influence bonding?
Who’s here for Ap chem?
you're the only reason why I'm passing! LMAO thank you
Glad I can help!
@@wbreslyn u r great
@@Raghav02 Hey, thanks!
Nice video So helpful to me
Thanks, glad I could help with the Octet Rule! --- Dr. B
YOU ARE AWESOME!!!!!!!!!!!!!!! THANK YOU!!!!!!!!!!!!!!
You are most welcome! --- Dr. B
Thank you very much dear sir
You are welcome, glad I could help with the Octet Rule! --- Dr. B
Hi idk if you see this but your a lifesaver
Happy to help!
Thank you so muchh
As a central atom (not counting free radicals), Group 1 - Alkali metals, Group 2 - Alkali earth metals Group 3 elements (3rd or higher period), C, N, O, F and Ne follow Octet rules.
C, N, O, F, Na, Mg have no 3d orbitals so no hybridation to break octet rules.
Bruh my teacher couldn’t explain me better than this guy did AND HE EVEN DID IT FOR FREE!
It would be good to have an explanation of why the octet rule works
Superb
Thanks 🤗
Can you explain it in chlorine's isotope
When you say minimize energy, what do you mean by that? I remember the term lowest energy state. Maybe that is a rule of nature. I am guessing that there is a whole lot more to this than could be. answered with a short answer. I have one more question. Hydrogen comes in pairs. Do the electrons circle the entire pairs or do they go and figure eights? I also wonder if the hydrogen atoms come together so that the electrons can interact with each other?
Good questions!
The electrons in a covalent bond are not actually circling the atoms in a figure eight pattern. Instead, they are spread out in a cloud around the two atoms. The shape of the electron cloud is determined by the shape of the orbitals that the electrons are in. So for H2 they are shared.
Yes, I would say the tendency of a system to minimize its energy is a rule of nature. In this case the H atoms molecule can minimize its energy by forming bonds between its atoms to make H2.
Ultimately, we’re all just looking for that special atom to come and stabilize us.
lol
When two Hydrogen bond together is it an octet or a duet bond?
I'd called it a duet. See czcams.com/video/R7SToNp1SO8/video.html. --- Dr. B
Thanks you
You’re welcome!
But the many body schrodinger equation REEEEEEEE!
Please make one video for duller rule also
Duplet*
Like this?
czcams.com/video/R7SToNp1SO8/video.html
Sir at 2:38 you made a mistake that Na is a metal and Cl is a non-metal. When you go to the right side of the group electropositivity increases.
Sir my Chemistry Mam teaches same like you. Thanks for the video.
I need a refresher, I slept during the discussion today 🤧😩
Would it be possible to have an octet for an atom?
Yep. All the Noble Gases have octets and they exists as individual atoms.
- Dr. B
thanks
You're welcome!
What about NaI?
Wow, I'm so clear bout ionic compound and covalent bond my professor cant explain shit
If your science teacher can't explain octet rule your kinda fuc*ed man
Nice
Thanks!
2 dislikes ? who the fck does that 1 like for you good sir thank you
well because there are people who don't care about school, and this video should have 13 likes, and 362 dislikes
@@candy5680 stop being an edgy middle schooler, be lucky he's helping you
Who the fuck r u to scold me he is dng it for money not for us and remember all r wasting time by not going to school
does octect rule work outsidide of earth
\
It should work anywhere in the known universe.
is Ionic bonds same as Ionic Compound?
That makes sense. Ionic compounds have ionic bonds holding the elements together. --- Dr. B
i know when atoms form ionic and covalent bonds, but when do they form metallic? is it metal + metal?
Also, in my chemistry course, we talk about polar and non-polar covalent bonds. What is this?
also random question: which sublevels are full when an atom has a noble gas configuration (test question)
Okay, so metal + metal is metallic bonding.
And:
Polar/NonPolar: czcams.com/video/OHFGXfWB_r4/video.html
s and p for nobal gas...
Hope you did well on your test! --- Dr. B
What about transition metal?
It gets more complicated with transition metals so we usually ignore them in general chemistry. --- Dr. B
Thx yo ur video explained a lot could u be my biology teacher
Excellent! Glad I could help with the Octet Rule. --- Dr. B
All professors need to use visual diagrams to explain this stuff
IB Chemistry been killing me
I see another deppresed ib student
IB is a tough course. Hang in there! --- Dr. B
Yes, my sleep schedule is non-existent. For the pass few days, I’ve slept immediately after school and woken up to do my homework at like 12 am