Aromatics & Cyclic Compounds: Crash Course Chemistry #42
Vložit
- čas přidán 26. 07. 2024
- What's that smell? Smell's like Organic Chemistry! This week Hank talks about Aromatics and Cyclic Compounds, naming their substituents, resonance, and common reactions & uses.
Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
--
Table of Contents
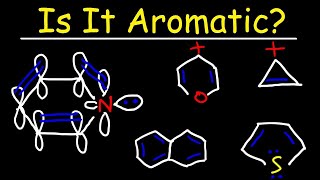
Cyclic Organic Compounds & Naming Their Constituents 1:06
Aromatic Compounds 3:02
Resonance 3:18
Naming Aromatic Compounds 5:05
Common Reactions & Uses 7:24
--
Crash Course is on Patreon! You can support us directly by signing up at / crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook - / youtubecrashcourse
Twitter - / thecrashcourse
Instagram - / thecrashcourse
CC Kids: / crashcoursekids








Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
Once again, we can find Hank Green saving my ass on a science test
Proud Hunter thank god for hank green :P
Proud Hunter hehehe me too chemistry#40 -#43 :)
"This is the same root as Bon Jovi."
OOOOOH!
"It's not."
...don't toy with my heart like that.
Hey, Folks! I believe everything he says so easily because of his credibility lol I’m so gullible
Last minute crash course before my uni exams as per usual
How did it go?
now back to ppap remixes...
+Max Jordan lololol
feel ya, bruh!
Nyaiffy I
Hank, you and John need to be in charge of all education in the country. Seriously, if my high school taught chemistry half as well as you, I wouldn't have been constantly failing.
"If you payed attention..." I love this ending to all the videos! Thanks, Mr. Green! (;
People are talking about uni exams whereas I just want to get through my high school chemistry test on hydrocarbons. XD
same 😂
I KNOW RIGHT. mine's like two days away
s a m e
exams are tomorrow aaaaaa,,
Haha same
ZozziFox i
thank you so much for this video :) could you continue the series and add a video about substitution reactions like the SN1 and SN2 and E1 and E2 ?
I really, really like the texture on this blazer. Looks soft and smooth.
LOL! Ikr! It was hard not to notice.
I just had 3 hours of lecture on aromatics and cyclics... yet, somehow, as usual, crashcourse took 180 minutes of convoluted information and delivered it in a fun, digestible, 10 minute informative video. Thank you!
I have been watching your videos for a month now and you helped me choose my major in college by showing me how awesome chemistry is. Thank you Hank!
It's so amazing how you cover exactly what I'm going over in Chemistry when I am being taught it. It happened with thermochemistry, electrochemistry, acid-base, and now organic chemistry. It's as if you made the videos specifically for me! Thanks for the awesome videos. :)
This video gives me flashbacks to Organic Chem as an undergrad. I drew so many aromatic rings that year...
What I've learned from this is that chemistry is batshit bonkers.
thank you so much I use crash course as a warm up before diving into the details. It's amazing how you guys script the information so both beginners and advanced learners can understand.
watching crash course before my tests as usual, thanks guys!!
I laugh at that Bon Jovi joke every time.
+Nika Cross Benjoin: Bon jovi, you can see where he's coming from
So thankful for these CrashCourse videos when exams are coming up. Definitely a concise but informative review of the overall topics. :D
I forgot how much orgo I forgot over the past 7 years since I last took them.
How long will it be until you forget that you forgot how much you forgot?
I won't find out that until I remember again
@justine klent madanlo lol
Learned more in this one video than in half a semester of classes, thanks :D
" just to make sure you're, like, good and solidly confused " XD
Really amazing.... Listening and visualising simultaneously in short timing is because of crash course only... And that historical stories ... I love it
Crash course video's like the ones on this channel are a good way to get to know the basics but it's a summary and therefore not detailed enough to pass your exams! For example the Huckle rule is not mentioned, just like the rules for aromaticy and anti-aromaticy and the fact that aromatic compounds don't undergo addition reactions. If Hank had to explain every detail it wouldn't be a crash course anymore but a college lesson. Just keep in mind that if you want to pass your exam, you better study from your books as well :)
that picture of drawing "Hank
dude...u speak so fast and clear!! ( compliment)
6:18 i was literally dying to know.
Congratulations on 200 Videos!
thankyou thankyou thankyou thankyou so much, this is just in time for my organic chem test tomorrow
You are doin great guys! It was really very useful.. and is indeed to get back to my subjects during exams making em more interesting...❤
These are great! Ya'll should be life savers and include organic reactions into the mix! I'll love that.
Thank you, this crash course was super interesting and helpful!
This was awesome, I think I am finally understanding Hydrocarbons and also all those chemistry drawings I have seen around.
Their videos have this very relaxing vibe which is the only reason i watch crash course not any other educational channel
seldom do i call my mom, but i got so damn excited about science i had to call her (shes a nurse so we have this love in common). you da man hank. and a thanks to all the guys at crashcourse
LOL, those wacky carbon atoms have me in stitches.
I finally get addition, substition, condensation and aromatics thanks to these videos. Thank you! (:
I AM A HUGE FAN OF YOUR VIDEOS. It makes boring topics so interesting and fun with your 3D visuals and Hank is so humorous as well. I WAS WONDERING, CAN YOU ALSO DO A VIDEO ON ISOMERSISM AND OPTICAL MERISM, THEN ALL MY CLASSMATES WILL WATCH IT TOO. Right now you skip over so many topics in a chapter, so they don't wanna even check you out. Please do that, you will be a great hit. I promise....
AH MA ZING !
This is what is so hard for our chemistry teacher to explain in 45 minutes, you nailed it!
I was quite literally just thinking about the science of aromas yesterday! Goodness. I still have a question: Does an aromatic material lose matter in order to give off a smell? Do incredibly smelly materials effectively have "half-lives"? Do radioactive compounds with half-lives have aromas?
when you smell something you are smelling the paricles that are light enough to get into the air (usually gasses) so no they are not radioactive or loseing material the material is just going into your nose and getting stuck. as far as particularly strong smells they usually contain certain atoms or molecules, nitrogen and sulfer being the most distinctive. nitrogen compounds are some of the worst smelling molecules with plesant names such as putrescine, cadaverine, and Spermidine... i'll leave it up to you to decide where that is from!
Awesome reference to Craig Benzine at 6:40!!
OMG! Hank you just made me fall in love with organic chemistry!!!!
Please please please do not stop doing this.... you are getting me through undergrad one video at a time....
also, I need next weeks video noowwwww
Should've talked about the 4n+2 rule
Huckel's rule
Yeah, ur right
This video made a mention to Jovi, Java, and Sumatera. Easily my most favorite CrashCourse video even though I love John's field more
Thank you for your video! You're the best! Just subscribed!
Amazing, thanks, love the vids.
I am in Organic CHM part 2 right now and man I wished I'd known these videos existed last semester! I almost didn't survive that class but you explain everything so well
thanks for this i really happy withthis video it helps me to take advantaged about all the compounds here..
Good refresh on my high school chemistry! Thanks!
Thanks for the educational video, it was definitely great.
Its so cool to understand the basic
These videos really help me to understand chemistry names.
These videos are amazing
This DESERVES my like
So beautiful, in every way. Thank you.
Wait. Hank has a degree in biochemistry, he knows about aromaticity. Being a ring with resonance is not where aromaticity comes from. Being a planar ring with p orbitals all the way around and having 4n+2 electrons in them where n is any integer is how a ring becomes aromatic. Other aromatic compounds include cyclopropenone (resonance structure produces 4*0+2 p orbital electrons), furan (oxacyclopentadiene) is aromatic (4*1+2 p orbital electrons), and borazine (4*1+2 p orbital electrons, and it isn't even organic). Meanwhile, compounds like cyclooctatetraene have the same kind of alternating sigma and pi bonds in a ring, but aren't aromatic. In this particular case, cyclooctatetraene actually bends itself out of shape because it has 4n (4*2) electrons, which would make it not just not aromatic, but anti-aromatic if it stayed planar. Anti-aromatic compounds are extremely unstable, so they tend to either not exist or find ways to avoid have a planar conjugated cyclic array.
in org chemistry i thought the term aromatic was used a little differently, and necessarily implies a carbon ring with resonance, so maybe he's trying to stick to the organic theme of this batch of episodes?
Thank you very much! This is really helpful! :D
Amazing!!!!!! My brain is melting
Great video :)
at 7:00 he seems so happy and theres the little guy on his shoulder like "ahhhhwwwww" And then he's like "It's not," with that smile I lost it!
happy 200th video crash course!!!!!!!!!!!!!!!!!!!!!
Now I am in Love with aromatics and cyclic compounds!
^_^ :')
+Mahir Muzahid me too!
Dude, get your freakin PhD and become a prof, you'd be amazing! This was a great way to review my biochem1 class 3 years later. I've been watching your videos for a while, great material, and it's great material I've been passing on to my students as well. Keep on keeping on!
Definitely a like on this one!!
Thanks for helping me study for my Organic Chemistry final.
I don't get why people complain about his speed, I always watch Hank in 1.25-1.5 speed. It makes more sense.
Cool
ty for speaking fast it saves my time..
Hank Green Blessed me !!!
'It's also the same as Bon jovi! Not really!' - Hank Green, 2013
In laymans terms for resonance. if the formal charge is imbalanced anywhere in the bonds, there will be resonance in order to balance this out.
For example: ozone which has one double bond. the single bond oxygen however has a formal charge of -1 while the center oxygen has +1, the third oxygen (which has the double bond with the center oxygen) has a formal charge of 0. because the balance with the center oxygen and the single bond oxygen is imbalanced it will switch the double bond between the first in third back and forth. O-O=O -> O=O-O the two possible outcomes of the ozone lewis structure. (sorry cannot show the additional electrons but this should help make things easier.)
huckel's rule and anti aromatic compounds deserved a mentioning !
You are a God send -_- I kid u not. Your always informative so nice to listen too cause your clearly passionate, keep it up! Thanks for the lesson ^_^
nice pics
+Peace John wow dude
good work
Wow...I didn't think it was possible to make a T-shirt and a suit jacket work together, but you pulled it off nicely.
Seriously though, I went from getting 40s on my exam's to 80s. Thanks Hank! You da bomb! Although, more organic chemistry videos would be nice.
+Logann Shepherd I say have a separate series of Crash Course: Organic Chemistry.
THANK YOU!
really love that sketch.....
helpful at the time of revision for Exams point of view......!
Thanks!
i just finish my organic chemistry B course, it was way more complicated than this, but it has lot of useful info in just 10 minutes :) nice chemistry snack
Was that guy with the coffee meant to be WheezyWaiter? Also known as Craig *Benzine* (which sounds exactly like *benzene*)?
it's so helpful 😃😃😃😃😃
You make me happy :)
Thanks to Ryan and Reese! :)
funny how he pronounced cyclic as sicklick :D thanks for saving me
I really need to watch every single episode of this chemistry course back to back and over and over again to learn these things, luckily I have a brilliant and cunning excuse not to, I'm fourteen and I am lazy.
What do you mean "Seriously?!", the phène/I illuminate thing is wonderful and brilliant. :)
Thanks
Despite their name, aromatics don't actually refer to their aroma. They were originally isolated from sweet-smelling oils but it now actually refers to their structures rather than aroma. I hope that's right as I just learnt it in A2 chemistry :)
Quantum-mechanical analysis of the MO method and VB method from the position of PQS.
The MO method and the VB method are analyzed using the principle of quantum superposition (PQS) and the method of describing a quantum system consisting of several parts. It is shown that the main assumption of the molecular orbitals method (namely, that the molecular orbital can be represented like a linear combination of overlapping atomic orbitals) enters into an insurmountable contradiction with the principle of quantum superposition. It is also shown that the description of a quantum system consisting of several parts (adopted in quantum mechanics) actually prohibits ascribe in VB method to members of equation corresponding canonical structures.
Using the quantum superposition principle, the MO method and the VB method were analyzed and it is shown that they are in contradiction with quantum mechanics. Also, using the quantum-mechanical description of a system consisting of several parts, it is shown that the attribution of canonical structures to the members of the equation is incorrect. Therefore, both the MO method and the VB method did not describe molecules with chemical bonds but actually, a lot of atoms (of which the described molecules consisted). That is, in the quantum chemical calculations, the chemical bond was "lost". Therefore, in order to "introduce" a chemical bond into calculations and avoid conflict with quantum mechanics, it is suggested to postulate the existence of MO as a new fundamental quality that describes a specific chemical bond and is not derived from simpler structural elements.
Quantum-mechanical analysis of the MO method and VB method from the position of PQS.
vixra.org/pdf/1704.0068v1.pdf
This is my favorite crash course. Make biochemistry videos
great video
who is watching this because of a exam i have one next week
Your making me want to study organic chemistry...
my chem exam is next week
Same
+Jay Bee good luck for us *fingers crossed*
same xD
Mine is tomorrow lol!
How did it go?
before khanacedemy addiction, and armando,, now crashcourse
SAME!!!!!!!!!!!!
The coolest thing about cyclopropane is that its bonds are called "banana bonds" (or bent bonds but this sounds more boring). Because the triangle has angles of 60° but its hybrid orbitals (sp3) have angles of 109,5°, so they overlap kinda bent - like bananas.
Only wish that Crash Course touched a bit on the mechanisms of a few characteristic reactions, such as Sn1 and Sn2 mechanisms for Nucleophilic Substitution etc.
Hank, could you make an episode
on Graphene. Thanks!
Where was this when I had my exam last Friday!! D:
But cool! :D
That Bon Jovi joke 😂. Oh Green 😪 hahaha!