IgA Nephropathy Treatment Options
Vložit
- čas přidán 9. 01. 2024
- Andy Udell, President of Calliditas Therapeutics North America, discusses IgA nephropathy and their Tarpeyo (budesonide) treatment. He also talks about Calliditas’ Phase 3 NefIgArd Study.
Immunoglobulin A (IgA) nephropathy is a rare, autoimmune, kidney disorder caused by the accumulation of IgA in the kidneys. IgA nephropathy typically has little to no symptoms in the early stages. A common early presenting symptom is blood in the urine. If left unmanaged, the disease can progress to end-stage kidney disease. Researchers have linked several disorders with IgA nephropathy, although the cause of the condition is unknown. These include cirrhosis of the liver, celiac disease, and HIV infection. Although IgA nephropathy usually does not run in the family, several cases of familial IgA nephropathy have been reported.
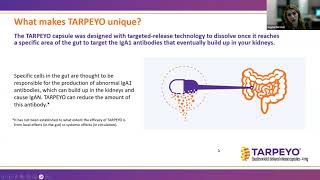
As Udell explains, previous treatment options for IgA nephropathy included using blood pressure-lowering medications and administering high doses of systemic steroids. However, researchers had not developed any treatments that specifically targeted the origin of the disease. With this in mind, the creators developed Tarpeyo by utilizing the active ingredient budesonide.
Udell also describes the NeflgArd trial, where researchers treated IgA nephropathy patients with Tarpeyo for nine months. In that phase 3 trial, patients were randomized to receive 16 mg/day oral capsules of Tarpeyo or placebNeflgArd trialo for 9 months, followed by a 15-month observational follow-up period without the study drug. The primary efficacy endpoint was time-weighted average of estimated glomerular filtration rate (eGFR) over 2 years. Upon observation, the researchers confirmed that the treatment provided a significant benefit over the placebo in the eGFR (e.g. 6.11 mL/min/1.73 m2 decline ie eGRF in the treatment group vs 12.0 mL/min/1.73m2 decline in the placebo group).
The US Food and Drug Administration (FDA) granted full approval to the drug in December 2023.








Commendable research and innovation indeed. I would like to ask if this is approved for the graft recipient.
Unfortunately Iga nephropathy is incurable and in 10-20 years leads to ESRD.
But progress can be prevented for long time by changing life style, exercise and less protein 😅😅
@@natvarsinhzala9078 progress cannot be prevented by any available means and sources, however, it can be slowed down with medication and good diet. Exercise is also OK but it increases blood pressure, potassium level and creatinin, therefore, only mild exercise is recommended!