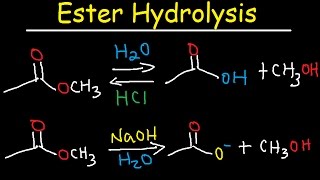
Fischer Esterification Reaction Mechanism - Carboxylic Acid Derivatives
Vložit
- čas přidán 26. 12. 2016
- This organic chemistry video tutorial provides the mechanism of the fischer esterification reaction which converts a carboxylic acid into an ester using an alcohol and an acid catalyst. This reaction is found in the carboxylic acid derivatives reaction section in your orgo text book. This video contains plenty of examples and practice problems. Molecules used in this video are acetic acid, ethanol, methanol and benzoic acid just to name a few.








Final Exams and Video Playlists: www.video-tutor.net/
can't believe so many professor get paid to do this while this guy does the teaching so much better.
Thank you so much for all of the work you've put into making your channel. The quality of your tutorials is unprecedented. I'm procrastinating the hell out of an organic chemistry lab report on Fischer esterification that's due tonight, as any good student would, and this video is a huge help.
I know it's been years, but I just have to ask: how'd the lab (and the rest of the class) go?
I love that you teach us these simple tricks about what product will be formed. I've been drawing out the full mechanism for each reaction, and while my professor requires that for a lot of the problems, this helps me know I've arrived at the correct answer.
i love how you draw the lone pairs out and everything so clearly! It's so easy to follow, thank you a millionnn !!
finally makes so much sense for the intermediate proton transferring part, i cldnt figure out how it jump that far till now i learned its from sovolysis deprotonate n protonate ! thanks a millions for the life saving vdo!!
What a lifesaver! I was having trouble understanding this. Thank you ❤
Thank you very much for the clear video! :)
Thanks for efforts. Really great explanation.
appreciate the time and effort!
have an o chem final tmr 😬 this guy is saving my life rn
Wooow thank you very much for translation and the wonderful explanation 🙏😍
i needed this, thank you so much as always
thanks for the video it really helped me in my extended essay
I can't believe that I understand the Ester formation thaaaank youuu😊
Thanks for the nice explanation...
According to my knowledge OH from acid and H from alcohol...
This video helped me solve o chem mcat questions
this video is bomb thank u
Thank you once again.
Where did you get the methanol from the second one tho?
Excess. There’s a lot of it
THANK YOU!
Could you have used the Cl ion from the acid catalyst to deprotonate the methanol attached to the tetrahedral intermediate? Or does it have to be the solvent that does this?
You can use the Cl-ion also
Fischer esterification? More like "Fantastic information!" 👍
I'm confused, do we need to remove OH from Alcohol or the acid?
Thank you. So the lesson here: if u don't want to fuck up your reaction, u better have quite an amount of excess alcohol. Increase H+ would be helpful to increase reaction rate to some extent.
u are amazing!!
Brilliant
Thank you sir
You're the best
Thankuu😍😍😍😍
Phenomenal
Where does the CH3OH come from?
why isopropanol reacts faster than methanol for transesterification?
thank you
I'm pretty sure HCl would ruin it. You need H2SO4, it's not a good NU like HCl.
Is it becuz HCl vaporizes or something? I remember that whenever I handle HCl, it smells very bad, so I have to do it in a fume hood.
@@raitodigital2092 Most classroom discussion of Fischer esterification use either thionyl chloride or sulfuric acid to start the reaction by protonation.
HCl will ruin it so you add pyridine to serve as a base and neutralize all HCl.
Yeah. The SO4- ion is pretty stable and remains a spectator ion unlike cl-
We love your videos! I think I will be able to make the exam just because of your videos! And my friend is your girlfriend now btw!
While removing water O of carboxylic acid is removed
have a organic chemistry final exam in 6 days. lets see how this goes
interesting.......
Thank you so much , but you didn’t talk about labeled methanol (O18)
not gonna lie, you had me in the first half...
Cool
❤❤❤❤❤
the voice quality is very slow in almost all your videoz
I am in love with you.
wtf
Cl- behaving as a base and forming HCl? I really doubt that.
Beside that very good video
My chem teacher said the same thing “Cl doesn’t really pull this H off but to make it easier we’ll say it does”
The maximum percentage of HCl is 37% in water, therefore 63% of the HCl solution is water and would shift the equilibrium to the left and form reactants over products.
Any Americans here, what grade would you do this type of stuff In America and what class? Brit asking here 🧐
@Pride oh my god really?! I’m doing this in my last year of a levels (age 18) before university. Would university typically be the first time EVER that you cover esterfication. Do you not learn it in like chemistry or AP classes?
@Pride oh wow that’s so weird. Thanks for the info!
@@redainaafridi4133 for real???? we literally learn this at the age of 16-17 in most asian countries for competitive examinations etc
In India you learn this in 12th grade for competitive exams.