Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32
Vložit
- čas přidán 28. 07. 2024
- Have you ever been to a Demolition Derby? Then you have an idea of how molecular collisions happen. In this episode, Hank talks about collisions between molecules and atoms, activation energy, writing rate laws, equilibrium expressions, reactions mechanics, and rate-determining steps. And funnel cakes are AWESOME!
Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
--
Table of Contents
Collisions Between Molecules and Atoms 0:00
Activation Energy 1:32
Writing Rate Laws 3:28
Rate Laws and Equilibrium Expressions 5:30
Reaction Mechanisms 8:06
Rate-Determining Steps 7:04
Crash Course is on Patreon! You can support us directly by signing up at / crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook - / youtubecrashcourse
Twitter - / thecrashcourse
Instagram - / thecrashcourse
CC Kids: / crashcoursekids








Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
good luck to everyone watching this the day before the test!
2019
Thx bro
*Day of the test
Thanks bro🙏🙏
🤝
Shout-out to Ms. Frizzle for winning the demolition derby
you won the comment section
She probably cheated. It's a magic school bus after all.
Literally crying tears of joy. I have a HORRIBLE chemistry teacher, who does not teach us (he just goes through extremely complicated notes and uploads extensively long videos), but expects everyone to master the materials. Tomorrow is our test on Kinetics and Equilibrium and THANK GOD FOR CRASH COURSE (and Hank) BECAUSE IT HAS TAUGHT ME MORE IN
Man, if crash course didn't exist, I would not be passing my chemistry class.
more like i would be passing out during chemistry class
I would like to thank Crash Course and coffee for my education...
This literally helped me understand kinetics more than my 3 months chemistry course at MSU lol.
These videos are a great review for my chemistry tests. I'm taking my second chemistry class (for science majors) as a summer course, so we are going through this information rapidly. I had to take it in the summer so that I would be able to take my MCAT at the end of my junior year. These videos have help me review, realize, discover, and remember very often. Thank you Hank and everyone else over there at Crash Couse!
“It’s kinda intense, but we can handle it. Check it out.”
Basically what I live by
Before I started learning chemistry I did a reaction with my friends where we put Lye into a bottle then add aluminum to it screw the cap on and it explodes we thought it was cool... but know I enjoy it even more because I actually know what's going on the aluminium acts as a catalyst and when you drop it in it produces hydrogen gas and steam pretty cool thank you Hank :D
this took cramming to another level
WHY DID I CHOOSE CHEMISTRY IM SO CONFUSED
I love science. ; - ; * ^ *
me 2
How is 0.060 is half of 0.012?
0.0060*
@@wasiangie hi
😂😂😂😂I missed that introduction sound. I haven’t watched a video for school from this channel in a long time. So happy to be back.
Who wants to have a crying session?
Fun AND intellectual all while teaching us the basics of kinetics? WHAT IS THIS BLACK MAGIC?!
no majiks r involved, just some people who know what gets us
It is the wondrous magic of Hank Green.
Also chemistry is f***ing awesome.
Yes of course, can`t you see the dark mark?
Hank Green....
....Bless you.
This video just summed up my last 3 chem lectures in less than ten minutes and made more sense doing it.
Who cares what grade you're in. Knowledge is knowledge, and grade (In this case) is just a number.
But if you're not old enough, you can't buy a bunch of bookcases and a Lamborghini to put in your garage.
How does he have the lung capacity for this?
dat sweet sweet editing
who knows who cares, i don't
He's mastered biology
This counts as studying for my chemistry exam next week, right?
Thank you for explaining concepts in such simple and beautiful ways!
dang if you play the video at half the speed Hank will sound as if he is totally drunk :)
+Ziyad Mohamed amazing
+Ziyad Mohamed That is brilliant! Much better way to watch the video my teacher assigned.
+Ziyad Mohamed lollll tru!
lol
Even the theme music sounds drunk
This is also relevant for college chemistry and I'm overwhelmingly thankful it exists! Thanks guys!
I had to teach this earlier, collision analogies and all - the day after the news of Paul Walker's fate became widespread.
It wasn't easy
I once watched this series for fun. I once again watched this series for fun. Probably a few more times to help understand certain things during my first university level chemistry class, did pass. Now I'm at the point of breaking down because the "orders" thing was explained by graphs and calculus. Hank Green saves the day, AGAIN.
He summarized the whole chapter of my book in just 10 mins. Wow!
I have a small bone to pick. The chemical reaction rate can be predicted by theory. There are variety of approaches out there, but Stephen Klippenstein and DG Truhlar of Argonne Nat'l Lab has written a variety of papers on the topic with various coauthors. See: The Journal of physical chemistry 100 (31), 12771-12800; Chemical reviews 106 (11), 4518-4584
Hi Hank,
I've been reading Six Easy Pieces by Feynman and he also states that atoms "bang into each other" but it is my understanding that it is an interaction of the forces between atoms which drives their movement and that nothing ever actually touches.
I understand that the language of the quantum world is in some cases inherently equivocal, but I was hoping for clarification on this topic.
Thank you,
Andrew
this is literally the only helpful resource i have found for kinetics. thank you!
I love this guy so much his teachings are 😍😍😍 I wish their was cashcourse math
There is crash course statistics
You are awesome as usual!! Thank you for the lesson. Love learning on CZcams.
I have a test in AP Chem tomorrow! This helped me a lot! Thanks!
@6:30 the lightbulb just came on! Thank you Green Bros. and Co.!
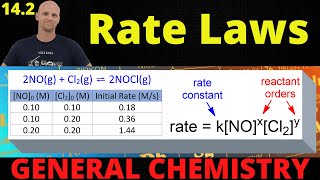
This video is perfect it gave me a great conceptual understanding of kinetic energy. The only thing I'm still unclear about afterwards is how to determine the exponential value of the reactants concentrations in the rate laws after performing the reactions experimentally and determining the rates of the reactions.
I learned so much from this episode, thank you!
Thank you so much! This is DEFINITELY the crutch I need to survive Chemistry!
It is the best video on kinetics . Thank you Hank and the team.
I would have 0 in chemistry without Hank.... Thank you ! I love you and appreciate your work.
This dude is saving my life right now. My chem professor is so monotoned I fall asleep almost every day. I don't feel so afraid of my test tomorrow now.
You make learning somewhat boring things very fun! Thank you for that! I also thank you for the little section center at the end of all of your videos! Thank You!
I think this is the first episode I have understood without having to go back and watch it.
I feel this is because I am finally understanding Chemistry, thanks guys :)
This helped very much! Thanks crash course❤ From India
Omg this helped me out so much. Thank you !
This was a very good refreshment on my Rates of reactions and such. Thanks hank.
this is amazing.. thank you a lot
this is amazing. Thank you!!!
studying this at school right now ! i love it when crash course aligns with my school syllabus :)
this was the most sexual first 3 minutes of a crash course yet
Hahaha genius..It got pretty gay at 2:15 though lol.
I know right, "So if chemical reactions only work when particles band into each other good and hard" lololololololol
i thought nobody noticed it
I thought I was the only one who noticed 😂
1Poiuytgfdsa1 Um.. biology has like 5 videos about reproduction, including human reproduction
Yes Chemistry is exciting and I came to know it through your lectures only Mr. Hank. Thanks :)
Cattle-lists, I geddit
It is still suggested to Read your text book . There are many equation stuff that were hard to explain in this short vid . After all this is just a crash course.
Good stuff I have a test on this on Thursday, good revision! :3
I love you Hank Green, I love you so much 😭 I have an AP Chem final this Monday and I am dyinggggggg
THANK YOU FOR THESE VIDEOS OMG
Amazing, as always! Thank you for these crash course videos, they really do help clear a lot of things up (and they're quite fun).
P.S. at 4:19 I think there's a typo, shouldn't it be "Amount of H2" instead of "Hz"?
I read a chapter of an Analytical Chem textbook on Kinetics, yet this video was more informative. Well done.
Great way to prepare yourself for college level chemistry before the semester starts. CrashCourse, you guys really do need to add a Crash Course Physics section!
You saved my ass. This was the one thing that was killing my chem grade.
Only if Crash course Knew about 10 min mark .
A catalyst actually provides an alternative pathway for the reaction with a lower activation energy, thus more molecules and particles have an energy greater than or equal to the activation energy, speeding up the reaction.
At 8:09' in describing the role of a catalyst, the common expression "lowers the activation energy" is used. A better description is that a different mechanism is provided through the catalyst and the activation energy of that pathway is lower than that of the uncatalysed reaction.
Thank you for making chemistry easy for me to learn
LOVE the breaking bad reference / You are amazing for including it :)
Well done! congrats to u all!
I'm looking forward to next week's segment on Solids, especially if it will cover the effects of surface area on reaction rate and other processes.
you have an amazing talent for teaching, i hope you have the success in life that you earn
these videos are awesome!!
Was the winner of the demolition derby a reference to "the magic school bus"?
Omg, I didn't even see that
Ms. Frizzle beat Sweet Tooth from Twisted Metal 😂😂
+Background Hero Media Of course she did, she has a magic school bus. Seems like cheating really.
+Background Hero Media "It's time to take chances, make mistakes, shut up and bleed!"
+Duke Travers Lol 😅
Magic School Bus is a win button in any Demo Derby since it can transform into anything you need. She could change it into a tank and wreck everybody.
the bus still can easily take a car down
Hank could be an auctioneer.
Great lesson overall. A small problem at 3:45' when describing the form of the rate equation. It is explicitly stated that only the reactants are included. When looking at overall reactions (macroscopic scale) there is no a priori reason to exclude products or catalysts in the rate law. It is common to have only reactants, and in teaching we tend to stick with this paradigm, but it is not the law.. A rate law that has a product with a positive reaction order will be "self-catalysing".
Why didn't I find this channel before? This is so good!
"If you paid attention" I feel called out 😭
i see Our Heisenberg in 4:09 and appreciate it, thanks thought cafe!!
Dang-it! Now I want funnel cakes!
Me too! D=
lulublondy But why does he hope they sold funnel cakes in chemistry class?
(I would understand why you would want a dessert in school, but why funnel cakes specifically?)
coolcatho13 Funnel cakes are sold at demolition derbies and he was comparing a demolition derby w/ chemistry class.
all the dislikes are the high school kids who don't like science
the words used to describe kinetics have changed my view on chemical reactions forever.
If only they would sell funnel cakes before the AP chem exam i have tomorrow :(
awesome video!!
this helped alot
It helped me understand the collision theory better, but the formula part just went over my head. Only hw practice will help me understand that at this point.
love that reference to the Magic school bus lol
Best videos out there!!!!!
@Jordana
I'd imagine the two go hand-in-hand in the manufacturing industry. Like, say, the conditions that favor the polymerization or formation of plastics or other important materials from their chemical raw materials.
Understanding batteries and similar power sources also requires a combination of chemical kinetics, as well as electrochemistry.
So I have taken a few kinetics courses and some of the information in the this video conflicted with what I learned class. To clarify: for elementary (single step) reactions the stoichiometric coefficients ARE equal to the rate law powers, kinetic theory and collision theory can be used to calculate rate constants and they are very close to experimental values for elementary reactions, transition state theory relates the rate constant to thermodynamics (everything must obey the laws of thermo).
Did Thought Cafe sneak Sweet Tooth into the derby? That was awesome!
Out of curiosity Hank, do you have any degrees ? I'd assume that you have a degree in chemistry or some form of scientific degree with how well you can explain concepts :)
thanks i wondered if that was the case
This is sick :D
I like your history lessons the most.
crash course is a blessing we do not deserve
Morning tea and chemistry. I feel like I should write a song about watching crash course.
48 hours later i will be taking a chemistry exam. Thanks to crash course for giving me a final tip!
How do you determine which step is the slowest? I had a question asking this on my hw and can't find anywhere that says how to figure it out
Demolition Derbys kick butt!
YES KINETICS THANKYOU!
just wanted to say crash course both chemistry and economics r real lifesavers.
I'm shocked you went with funnel cake instead of fair corndogs, Hank!
Also, nice Equilibirium reference.
Anyone else curious what Hank's shirt says?
It says- eat five sheets of toilet paper while discussing the political situation in Nepal
At 4:30, you said that to double the hydrogen you raise the exponent to the power of 1. However, raising anything to the power of 1 leaves it the same. Can you please explain this?
8:23 Cattle List... I found that visual pun amusing.