I3- Lewis Structure - How to Draw the Lewis Structure for I3-
Vložit
- čas přidán 24. 05. 2013
- A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide Ion).
For the I3 - structure use the periodic table to find the total number of valence electrons for the I3 - molecule. Once we know how many valence electrons there are in I3 - we can distribute them around the central atom with the goal of filling the outer shells of each atom.
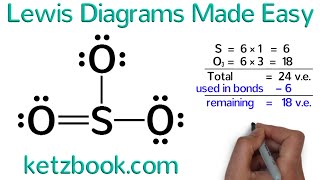
In the Lewis structure of I3 - structure there are a total of 22 valence electrons. I3 - is also called Triiodide Ion.
Note that I3 - can have an Expanded Octet and have more than eight valence electrons. Because of this there may be several possible Lewis Structures. To arrive at the most favorable Lewis Structure we need to consider formal charges. See how to calculate formal charges: • Formal Charges: Calcul...
You should put brackets with an negative sign around the I3- Lewis structure when you are finished drawing the structure.
---- Steps to Write Lewis Structure for compounds like I3 - -----
1. Find the total valence electrons for the I3 - molecule.
2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside.
3. Put two electrons between atoms to form a chemical bond.
4. Complete octets on outside atoms.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
---- Lewis Resources ----
• Lewis Structures Made Simple: • How to Draw Lewis Stru...
• More practice: • Lewis Dot Structure Pr...
• Counting Valence Electrons: • Finding the Number of ...
• Calculating Formal Charge: • Formal Charges: Calcul...
• Exceptions to the Octet Rule: • Exceptions to the Octe...
Lewis Structures are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Triiodide Ion. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule (like boiling point and surface tension).
Chemistry help at www.Breslyn.org








Thankyou for doing SO MANY lewis structures unlike others!
did u graduate
Cheers mate!!! Thanks a lot!!!
Your 8 year old video helped me now
Thanks man
bro you're the best
Ya
Sir, can you please explain the formation of KI3 and the oxidation state of I in this case
is resonance possible?
O Brasil agradece !!!
thank you so much.
We consider the two iodides bonded as 1 when calculating the formal charge ?
THANKS :D
how many lone pairs are there?
In my notebook, it is said that if there is a negative charge on ion then it will be on corner atoms but here negative charge is on Iodine (Central Atom Iodine), why?
There are exceptions to most every rule in chemistry. Here the central Iodine atom has two lone pairs of electrons which leads to the charge.
i love you dr b
in I3- which is the lewis base?
sir my teacher said KI3 is having coordinate bond. can you please explain?
You are awesome!!!!
How do you use formal charge to help confirm the lewis structure?
Take a look at my website for information and a video on formal charges. --- Dr. B
www.thegeoexchange.org/chemistry/bonding/Formal-Charges/index.html
Wayne Breslyn Thank you so much! Your videos are very informative and they're such great resources for studying.
Please explain.
Central Iodine atom doesn't obey octet rule, Why?
+Kumaresh Sekar Central atoms that are from period 3 and beyond can expand their octets and have 18 valence electrons and this is mainly due to the fact that the 3rd main energy level has sub shells that are close in terms of energy.
@@renedescartes477 the 3rd main energy level has sub shells that are close in terms of energy, really?
This question asked in jee advance 2019
cool
has central iodine 10 electrons (an exception to octet)?
yes
GOOD .
i love you man
why not form double bonds instead?
Because forming double bonds would change the formal charge of the atoms. Check his video on formal charges for conformation. www.thegeoexchange.org/chemistry/bonding/Formal-Charges/index.html
I think because the other *I* has its octate complete already
Sir, what about its polarity? Some says it's polar, some says it's not. In my opinion it should be non polar, but want an answer from your side...
My sense is non-polar but this is complicated by it existing as a crystal and when in water, an ion.
This discussion is interesting:
www.quora.com/Is-I3-polar-or-non-polar-What-makes-it-so
How many loan pair electrons?
I get 18 lone (like alone) pair electrons. We don't count the pairs between atoms. Those are the ones that form the chemical bonds. --- Dr. B
why doesn't it form a double bond?
I wish I knew! @waynebreslyn?
Because forming double bonds would change the formal charge of the atoms. Check his video on formal charges for conformation. www.thegeoexchange.org/chemistry/bonding/Formal-Charges/index.html
I have been wondering this too. YoLO AcE you know that is absolutely useless in this situation, have you tried that approach? If you have you'd know the net FC of both double bonded and what Dr. B did, come out to -1. What we are supposed to assume here is that the non-double bonded one has more octets than the double bonded version. I could be wrong here, but I know that FC has nothing to do with it, unless there is supposed to be an even distribution of the FC (like on the double bonded version the -1 FC is on the outer Iodine, whereas the -1 FC is on the central Iodine in Dr. B approach).
It seems like the central atom is the one that should be able to break octet rule. So I think maybe that is what a large mass of people are running into when they make this.
sir can you explain this lewis structure concept for [XeF8]2-
Interesting, I never hear of that one. You have a total of 66 valence electrons (8 + 7(8) + 2 = 66). Xe can have an expanded octet. I'd put the eight F atoms around Xe with a pair of electrons shared and see what happens. --- Dr. B
Note: en.wikipedia.org/wiki/Nitrosonium_octafluoroxenate(VI)
formal charge on xenon further decreases when it accepts electron from environment. Xe=0 to Xe=-2.
My guess is that Xenon is a big enough atom that it doesn't hold electrons too strongly which is why F can form bonds with it (F being very electronegative). --- Dr. B
thank u so much sir.
개지렸습ㄴ다
This question asked recently in jee main 2018 .I lost it ..... feeling bad available in u tube
No of lone pairs pucha tha
Waht was the original question??
How many lone pairs
I'm counting nine lone pairs for this Lewis Structure. Remember, lone pairs aren't shared between two atoms. --- Dr. B
Does it form covalent bond...?
Yes, it does. These are all non-metals which leads to a sharing of electrons, a covalent bond. --- Dr. B
Thanks.. how to identify covalent bonds..? Is there any trick...?
There is a pattern, to be sure. Take a look at this video:
• Types of Compounds: czcams.com/video/Nm-2HIrca2s/video.html
This might help too:
www.breslyn.org/chemistry/naming/
@@wbreslyn Does it form a coordinate covalent bond (also known as dative bond)? How to identify if a species has coordinate covalent bond?
Why is it linear instead of see saw
That's because it is a symmetrical molecule.
This might help: en.wikipedia.org/wiki/Triiodide
--- Dr. B
Can this form resonance with a double bond I understand it would want to make one; but CAN IT, i know teacher is going to ask for resonance on this.
I don't think I've seen a double bond between to elements in Group 17. If it were possible, the formal charges wouldn't be favorable and it would be a minor resonance structure. --- Dr. B
Thanks this is one I believe she is toting in class I believe she just teaches it as if it is in period 3 or higher then it can have an expanded octet, so therefor resonance is no problem with a structure that has 3 lone pairs. I'm assuming if she asks for resonance she isn't looking for me to say "no resonance" and I looked through my notes it is just the criteria that qualifies it as a resonance; I think she picked it as you can create soooo many resonance structures by removing any lone pair on the the terminal atoms, obviously they cant be changed from the central atom as that would change it's molecular geometry. However, I am with you I haven't seen it with a double bond I am assuming that is because halides would technically only want to make one bond althought it is still possible, I hope she isn't that mean, and when I draw the resonance structure with a double bond she doesn't mark me wrong.
I'm guessing she'll have to mark a bunch of other people wrong at well!
Is there any way to draw a resonance structure for this?
I don't see any if you want to maintain the molecular geometry. But otherwise you could shift the electrons around in a number of ways by having the expanded octets on the outside Iodine atoms. You could probably even do a ring structure, although it may contain double bonds.
is there a dative bond?
dative bonds means coordinate bond?
is there a dative bond?
in I3- which is the lewis base?
Isn't every I atom supposed to have 7 electrons in their valence shell why does the one in the middle have 8????
Jeet bhai
Bad bad worse