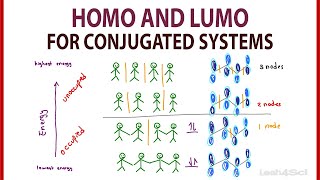
Introduction to the Molecular Orbitals of Conjugated Alkenes
Vložit
- čas přidán 13. 04. 2013
- Professor Davis says a few words about the geometries and energies of pi molecular orbitals in conjugated alkenes, using a the classic examples of ethene,1,3-butadiene and 1,3,5-hexatriene.








This is the best explanation on molecular orbital I have ever seen 👍
I don't understand why these don't have more views! They are incredibly well done.
This is by far the best tutorial I've found (after many hours of searching youtube) about Conjugated pi orbitals. Thanks a lot !
Mighty Ashur Thanks so much for the kind praise!!! Please share and subscribe!!!!
agree with u
honestly yeah I spent hours searching too. watched many vids. this the best.
Wow, I really wish our professors had such beautifully crafted presentations. Makes even Khan videos look old ;-)
Thanks, Reto. They take a tremendous amount of work, and it is wonderful to know that all of that work is paying off!
So logically explained and well presented! The 3D pictures were EXTREMELY useful. Even though my professor lectured this topic for 2 class meetings, I am just now understanding the material after watching this video. Thank you so much!!!
+Aida Ibrahim Thanks for the kind words. If you like my teaching style, I suggest that you take a look at my entire course, available at www.thegreatcourses.com/courses/foundations-of-organic-chemistry.html
You saved my life! I have spend days understanding this! thank you so much!
my teach skipped a MO description of conjugated alkenes; thanks for the satisfying explanation.
Glad you found it useful! Please remember to subscribe, like and share!
This was so helpful! It used the same examples as Wiley's Ochem and it was a great companion to chapter 17. Really helped me understand vs just reading and looking at pictures flat on a page.
Thank you very much ...i ACTUALLY UNDERSTOOD THIS MATERIAL!!
This video makes so much more sense than the textbook. Thank you!!
thanks, Alex! I'm glad you found it helpful. I think the color and especially the animation are just not possible with a text book (great though those resources are!). Don't forget to 'like' and pass the video on to your friends and classmates.
ChemSurvival
Hello Mr Chem. :) How cus you know for sure electrons exist? I have heard many many many many people say that they do. But I don't understand how we arrived at this point? What is it, that lets us KNOW, that electrons are, objects, and not fields with certain thresholds?
Some of the best evidence we have is over 100 years old. Try reading a bit about the Millikan Oil Drop experiment
Millikan Oil Drop Experiment
because all of the charges Millikan observed were integer multiples of his base value, he basically proved that electrons must be distinct particles, each with a charge of -1.6e-19 C. Otherwise, charge would not always manifest itself in integer multiples.
Thanks for sharing! having gone to lecture and reading the chapter on conjugated systems I was still a bit confused, your video made everything I was reading make perfect sense,
Thank you.
Extremely helpful video ! The instructor at my school presented this information in such an overwhelming manner. Thank you !
I'm so glad it helped you. The best thanks you can offer is to share my video and channel with your classmates and instructors. Cheers!
Lecture is osm thnks alot sir 💐💐
Thanks for the molecular orbital lessons, I really wasn't understanding how my prof explained it.
you saved my life. thank you!!!!!!
awesome video!! this really clears things up and is a great explanation of the mo theory of conjugated dienes!! thank you so so so so much!!!!
Liked it, helped me understand how to find the MOs of alkenes and etc.
Lots of love from Pakistan Amazing sir ...
By far the best explanation. Thanks a lot!
Thanks!!!
Thank you so much!!!! I understood everything!!
You have saved my life !!!
Thank You, that was so good
Clarity level really high
I swear 90% of my college degree is from youtube & I swear it's because of ppl like you.
Thanks for the kind words. The greatest thanks you can possibly give is to help me share my channel with your friends, colleagues and instructors. Good luck with your studies!
How am I just finding this channel?! Subscribed. Great content. Just in time for me to start o-Chem 2
Thanks for the sub.! Don't forget to share with your classmates :-)
You are slaying this.
best explaination ever.Thanks sir
Thanks for making this! Molecular orbital theory always makes my head spin but this helped a lot.
Glad it helped! The greatest thanks you can give is a like and share 👍
Thank you very much !!!!!!!!!! it is really interactive!!!!!!!!
Hadir tanda support
Very helpful,thank you sir.
thanks for helping me in understanding the topic
Very helpful, thank you!
Thank you so so so much!!! This video made my life so much easier in understanding homo and lumo.
Glad you found it helpful! Please subscribe, like and share with your classmates and friends!
I did!! I've already sent this video to a couple of my friends hehe :) thank you so much, i look forward to watching more videos!
Totally brilliant!
SMOOOOOOTH!!
Now I need the mathematical calculations using LCAO! Got a video for that yet?
Thank you so much! I was so confused
it was very good..
wow perfect explanation. i learn alot from this video thanx aloooooooooot :)
Glad it helped! It is part of a course that I just produced with The Great Courses! It will be out very soon. Find more information at the link here! www.chemsurvival.com/TheGreatCourses.html
YES! You champion.
YOU R GRATE PROFESSOR...:
Amazing!
thank you very much sir
Fabulous best
amazing thank you
wew! why can't they have these kind of explanations in the books.
Thank you, thank you, thank you.
You are welcome! :-) If you really want to thank me, please subscribe to my channel and share this video with your friends, classmates and colleagues!
Thank you for the good explanation. What I dont understand, can't there be more opttions of MOs. For example with 1,3,5-hexatriene cant there be the following constellations of the blue part of the orbitals: up-down-up-up-up-down or up-up-up-up-down-up? Do those versions not exist and there are actually only 6 possible MOs for 1,3,5-hexatriene or are they just irrelevant and if yes why? Because up-up-up-up-down-up only has 2 knots so shouldnt it be around pi3 at the energy level?
Would really really appreciate it if you can also make a vid of pi systems with non bonding electrons. Still unsure how to draw the MO or where should i put the nodes for the nonbonding MO
thank you sooo much!!!
glad you found it helpful! Please pass the video on my channel along to your friends and classmates
Thanks.
Soooo, does this mean that there are many sub-types of a molecule besides the obvious differentiations (stereoisomers and such)? Do these nodes give rise to different properties amongst the apparently same molecules?
Not quite Eric. The many sublevels all belong to a single molecule. electrons can transition between and among all of these levels in a given molecule even though we typically focus on the pi to Pi star transition because it is usually the most prominent
This is fucking good animation of how electrons move , I just know it from now
Sir why pi 1,2,3 contains only 2 electron.Why the all 6 electron of haexa tri ene is not placed in single step,suppose the lowest energy step pi 1
If I have a 1,3,5-heptatriene, will it be 7 M.O then? Or do I just have to consider only the six Carbons involved in the double bonds?
Remember that these are pi M.O's. Only the six carbons involved in double bonds are considered for the pi system. The terminal carbon in 1,3,5-heptatriene is sp3 hybridized, so it does not participate in the pi system WHEN THE COMPOUND IS NEUTRAL. If instead it you are dealing with 1,3,5-heptatrienyl cation, (or anion) the empty (cation) or lone pair (anion) orbital on the terminal carbon is now fair game, and things can change!!!
thanks
You're Welcome!
Thanks a bunch. You literally saved my neck this semester. But can i know why Non- Bonding orbitals havent been mentioned??
Thanks for the comment. In this highly simplified and focused example, non-bonding isn't an issue, since there are no energetically neutral M.O.s. However, such M.O.'s do surface in other simple examples, such as simple aromatics, which I cover in this lecture....
aromatics
czcams.com/video/PkzN4PGN2ro/video.html
In case of Butadiene, isn't there another possible permutation, i.e. + - - - ?
I thought so at first too, but here's a line from one of my text books "For a linear system of conjugated p orbitals, all nodal planes in a resulting π MO must be positioned symmetrically about the center of that set of p orbitals." It has something to do with quantum mechanics. So yeah, only took 3 years for the internet to answer your question.
Can someone tell me, how it is that we know electrons exist? B/c all of my theories, which i made in my head, say thy shouldn't exist. & I want to know if I'm right or wrong... so how is it again, that we KNOW that electrons exist?
I think therefore I am my friend, if you do not believe it exists, you know it doesn't.
i am not able to understabd structure over all very nice
where is non-bonding MO.?
This is from GOD!!!!
Your subtitle is disturbing the diagram
as as as
uh
Very helpful! Thanks!
My pleasure! I'm glad you found it helpful!
Thanks you so much, very helpful!
glad it helped!