Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds
Vložit
- čas přidán 6. 03. 2021
- Leah4sci.com/MOtheory presents: Molecular Orbital Theory for Sigma and Pi Bonds
Need help with Orgo? Download my free guide ’10 Secrets to Acing Organic Chemistry’ HERE: leah4sci.com/orgo-ebook/
In this video, I’ll walk you through the most basic and foundational concepts behind Molecular Orbital theory for both sigma and pi bonds. You’ll learn the difference in bonding and antibonding molecular orbitals through a simple analogy and also how they compare on an energy diagram to the atomic orbitals of the bonding atoms. Additionally, you’ll learn to depict molecular orbitals, both bonding and antibonding, with simple drawings.
In this video:
[0:13] Introduction to Molecular Orbital Theory
[2:29] Review of Energy Diagram of H2 Gas
[6:04] Difference Between the Antibonding and Bonding MO
[7:10] Molecular Orbitals for Pi Bonds
Links & Resources Mentioned In This Video:
Organic Chemistry Basics Leah4sci.com/OrgoBasics
Resonance Structures in Organic Chemistry Leah4sci.com/Resonance
Molecular Orbital Theory Leah4sci.com/MOtheory
This is part of the Molecular Orbital Theory Mini Series. Catch the entire MO Theory Video Series on my website at leah4sci.com/MOtheory
Follow along with your semester by using my Orgo Syllabus Companion: leah4sci.com/syllabus
For more in-depth review including practice problems and explanations, come join my online membership site the organic chemistry study hall: leah4sci.com/join
For private online tutoring visit my website: leah4sci.com/organic-chemistry
For questions and comments, find me on social media here:
Facebook: / leah4sci
Twitter: / leah4sci
Instagram: / leah4sci
Pinterest: / leah4sci






I am not exaggerating, but This Video is the best definition of being underrated. One of the perfect analogies I have ever witnessed. Wow. Simply Wow.
Wow, thanks so much for your kind words!
ratio
Fr
I absolutely LOVEEE how you explain every single topic !!! Thank you so much you don’t know how much people you are helping with explaining such complicated things in the easiest way. You are amazing ❤️❤️❤️
You are so welcome!
Thank you for being so calm and patient
You're very welcome!
Thank you. You made anti-bonding crystal clear.
You're very welcome, happy to help!
I have watched several videos to try and understand this concept, and yours is the first one that clicked! I finally get the concept of bonding and anti-bonding molecular orbitals- thank you!!
Wow! So glad that this helped it click for you!
I graduated from pharmacy college last year, you helped me a lot in Organic chemistry through out the years,
so thank you a lot.
You're very welcome. Congratulations on graduating from pharmacy college!!
Leah, I LOVE YOU... your videos are something everyone needs to see chem student or not!!!
Awww, you're so welcome
that is exactly what I want to find. Simple and thoughtful. Amazing explanation. Thank you
You're very welcome!
You are a LIFE SAVER!!! You have to fully master a subject to be able to explain it in a clear, simple manner, and you have proven yourself a professional via this video! THANK YOU!!!!
Wow, thanks! I'm so glad that it helped you so much!
Wow this was I needed
In my college they taught this last week
So for preparation I needed
Thank u
Talk about perfect timing! I'm glad the video came when you really needed it.
Hi Leah, thanks so much for your videos they really helped me get into my top choice university undergraduate program. All going well I'll start at Oxford in October. Keep up the awesome content!!
Oh wow that is absolutely amazing!! Congratulations Nikolas!
@@Leah4sci thank you so much!!
I love LOVE LOVE!! the way you explain. God bless you, seriously. Thank you so much!
Awww, you are so welcome!
I truly cannot put into words how much I appreciate these videos. Things are FINALLY clicking for me
Yay, I'm so glad to hear it! Use my syllabus guide to help you match all my resources to whichever topic you're working on: leah4sci.com/syllabus
Literally amazing analogy so easy to understand now- and easy to recall the concept with this way of explanation
Great to hear!
Alongside a beautiful voice, that was such a brilliantly simplified illustration. Pleasantly clarified the whole mystery of a Molecular Orbital existing in 2 forms at the same instant. Thank you, ma'am. Wish the world had higher intelligent beings like yourself to question the basics and have a broader outlook. The Big Picture. LOVELY
Awww, thanks so much for your kind words. I'm so happy to hear that my videos are helping you to understand organic chemistry better.
Down bad.
Thank you, this was very helpful :)
You're very welcome!
Loved it ...
Made me understand it in minutes
Thank you
Glad it helped!
I am regretting for not watching your videos during my first years when I was struggling with hybridization. Keep going your explanation is the best 💞
So nice of you! Thanks for watching.
Leah, gal, woman, may you be blessed with health, wealth & time to enjoy it...absolutely fabulous lesson
Thanks so much, and glad you enjoyed the lesson!
Have no words to thank..have been searching for the best explanation of MOT in youtube then got blessed by this❤😊
Awesome, I'm thrilled to hear how much this is helping you!
Wow.
Wow....love the people analogy! I m really starting to understand this topic! Thanx!
Happy to hear that you're understanding!!!
Thank you so much for chemistry easy to understand and teach for us .
You're very welcome. It's my pleasure!
Thank u so much for such a great explanation ...u made it easy 👍
Glad to hear that. You're so welcome.
just what i needed, thanks a lot, no kidding, best explanation ever!!!
Great to hear! You're very welcome.
This was such a great video, thank you. Studying for my biochem midterm right now and was very confused on this
Glad it was helpful! You're very welcome.
Amazing video👍 nicely explain.
Thanks so much! Glad you enjoyed it Anup
My Leah!😊
Thanks so much for this☺️
You're so welcome!
I had some difficulty in understanding this,till I watched this video!.
Thanks Leah!
Glad it was helpful!
Thank you so much for this 💐
You're very welcome!
This is the best video I have found to date. No joke.
Wow, thanks for the compliment! Hope the video helped. :)
Definitely deserved thumps up
thanks :)
This video the content on your website help me immensely with self-teaching.
Glad to hear it!
Very well done!
Thanks Debra
🤓 thanks! Wanted clarification for antibonding
Antibonding is when the electrons have very high energy and separate from each other rather than forming a bond. Meaning, they don't exist in the region between the nuclei where you'd expect to finding bonding electrons.
I like to think of it as a couple that is very angry with each other and break up
Thanks for clearing my doubt
Happy to help clear things up for you!
great explanation 💗💗
Glad it was helpful!
Hi Ms Leah, I enjoyed your explanation and the expressions of the cartoons. Thanks, Kasi
You are so welcome!
Just perfect 🔥
Thank you!
You made optical isomerism very easy to understand 😀
Glad you found my methods easy to understand :)
@@Leah4sci 🥰🌸👍
Nicely explained ❤️❤️
Love from Kashmir
Thank you! Love back from NY
I have to make a PPT for this topic but i am not good in chemistry but now i think i can make it this video is really helpful
Awesome, happy to hear it!
Great video!
Thanks!
So good thank you
You're welcome!
you are seriously saving my a$$ rn tysm
Happy to help!
Electrons: a romantic comedy. Seriously, it makes me more confused when professors bring up MO theory , antibonding orbitals, etc, but don't explain how it's applied to conjugated /aromatic systems behavior...it drives me bonkers....makes everything more confusing. I needed MO theory in plain English and you delivered big time :)
haha yup! I find that if you given chemistry/reactions human characteristics, everything suddenly makes more sense. Glad it helped
That was my same problem when I was taking Orgo. They always bring up anti-bonding and bonding but never explain it in essence.
Best Tutor Ever❤
Aww thanks!
What I've learned halfway through the video so far!
There are atomic orbitals, which belong to unbonded atoms, and molecular orbitals, which are shared between bonded atoms.
Within molecular orbitals, the electrons can either engage in constructive interference or destructive interference. This can also be described as lower energy, stable bonding orbitals, or higher-energy, more unstable antibonding orbitals.
And... time to watch the next half of the video! :)
Part two of the video!
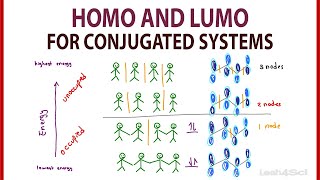
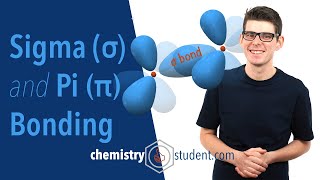
Sigma bonds are simple overlaps, pi bonds are a different shape above or below the atoms. They have a node in the middle where the pi electrons do not go. The antibonding pi orbitals have a big node/separation between them, making them higher energy, whereas the bonding pi orbitals overlap and have a tinier node.
It's like a relationship. If they are angry and high energy and separated they are antibonding and there is no overlap between the pi orbitals and they are unstable. It's like the pi electrons are only on one of the two carbon atoms, giving them different charges and making them unstable.
If they have lots of closeness and no distance between they are happy and cuddly and close and stable and low energy! The pi electrons are both equally shared and there are no partial charges.
And... time to learn about HOMO and LUMO! Thanks for another great video Leah.
Glad you're learning so much!
Finally I got to know how free radical separation works... thanks!
Yay! So happy that you understand, and you're so welcome!
Was looking for the physical approach and got super excited.. for 2 seconds xD
Thanks for watching, anyways! :)
I have benefited a lot, thank you
I hope my doctor will teach us like you
Pharmacy student from Iraq
I'm glad you benefited from the video. And if your doctor doesn't teach this way, come back to my channel for more :)
you the man homedog
Happy to be 'the man'! Lol
Heyyy
I love the explanation and I’m surprised to see that you have literally replied to every single comment.
You really care about the viewers.
Well I have chem final in 1 day and I’m super scared. 😢
But yea keep us teaching ❤❤❤
I love to stay in contact with my viewers! Thanks for watching, and best of luck on that Chem final!
If you find you're looking for more help, I recommend joining my organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
@@Leah4sci well I will see in my next year(senior rn I’m a junior)cause rn after this final I will have to prepare for other finals too.
Thank you for your support and efforts ❤️
Hyeee,,, your method is amazing,,,
Thank you
@@Leah4sci welcome😊
excellent excellent excellent...
Thank you so much 😀
SUBSCRIBED!
Thanks! I appreciate it!
Constructive interference means that the two waves that are in phase add together to form a giant wave and conversely destructive interference means that the 2 waves which are out of phase are cancelling each other out and you get no wave so we can think of an electron as wave or a particle.
Thanks for watching! :)
You are an amazing teacher. The way you teach us is very simple and easy to understand. I found your channel and very thankful to you ♥️. I want some more videos from you other than organic chemistry, like other chemistry topic. Your all videos of orgo are just love. You helped us lot. You deserve more than this. Always support and love for you ♥️. 🤗 Looking forward for your response. 🙂
Thanks for the feedback and suggestions Sohag
you saved me Leah 😊
So glad to help!
antibonding always seems counterintuitive, that node makes sense though because the signal or wave can be continuous still even if it cycles
Thanks for watching!
Hlo mam your teaching method is awesome and it helps me to revise concepts fastly and easily why not you start teaching for Indian exam JEE please ,and lots of respect and love from india♥️♥️♥️
Thank you! I'm not familiar enough with the JEE exam
@@Leah4sci no problem mam you keep on teaching us in the way your teaching 🥰🙏
i luv this woman
Thanks!
Can someone briefly explain hyberdrization? I thought i understood it (2 bonds for sp, 3 bonds for sp2, 4 bonds for sp3) but there seems to be exceptions, so i'm not really sure why those happen
I cover it in detail here: leah4sci.com/HYBRIDIZATION
Can you explain SO³ to me? Is it shaped more like a shuttlecock or do the oxygen flare out to a more perpendicular shape, when they are compared to the Sulphur-S lone pair axis?
I'm sorry, but I don't offer tutoring over social media. For help with questions like this and more, I recommend joining the organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
can i ask a quastion? why antibonding has a higher energy level than bonding? i already search article but i cannt understant can u explain to me? Thx a lot
It has to do with the presence of the node between the nuclei of the two bonding atoms. The antibonding orbital is higher in energy because of the decrease in electron density between those nuclei. In other words, the electrons have less freedom of movement in an antibonding orbital and remain partitioned to either side.
Super mam
Glad you like it!
Just to clarify madam, do anit bonding and bonding orbtials happen or exist at the same time in the same 2 atom? Eg. If i have 2 Li atoms will their bonds have both antibonding and bonding orbitals at the same time?
The energy within a bond is always fluctuating and so the electrons will move between bonding and anti-bonding
' double bond
in C2 consists of both pi bonds because of the
presence of four electrons in two pi molecular
orbitals. In most of the other molecules a
double bond is made up of a sigma bond and
a pi bond' can someone explain this a bit simpliefied , does carbon molecule have 2 pi bonds between them because it have 2 electron each in bonding pi orbital , and no extra electrons in bonding sigma orbital and antibonding pi orbitals
The carbon-carbon double bond shown in the second example of this video has a single pi bond and a single sigma bond. The pi bond is made of p orbitals that sit both above and below the sigma bond, in a single plane.
For help with questions like this and more, I recommend joining the organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
So I need to learn higher math for a better understanding then the analogy?
Yes. This video only covers the very basics so that you know what you need to know for a typical Organic Chemistry course.
thank you for making horrible topic ,beautiful. Analogy of persons with bonding and anti bonding is superb.
You're welcome; I'm so happy to hear that I've helped you love the topic!
I sort of like this, but don't understand why there can't be one electron in each hydrogen molecule orbital.
It might simply be an unlikely and essentially unstable configuration, no more stable than two separate hydrogen atoms, so just the same as having both electrons in the antibonding orbital.
When the 2 hydrogen atoms each have one electron in their respective orbitals, they are lone H atoms, unstable due to being unpaired. However if they come together at high energy you get the antibonding molecular orbital (vs the single electron atomic orbital)
🙏🙏🙏
Glad it helped!
11:48 kinda scary 😳 and interesting 😅
lol
Hi
Hhhhhhhh
Hello? Thanks for watching. :)
Hi! What would an example of something that would case the H-H to go from the low energy molecular orbital Tom the high energy molecular orbital
I'm sorry, but I don't offer tutoring over social media. For help with questions like this and more, I recommend joining the organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
Very scientific start of the video. Know your limitations, lady.
Thanks for watching anyways!
final year of university and i still cant grasp this one concept
I'm sorry to hear that! For more help, contact me here: leah4sci.com/contact
I have adopted you as my primary teacher in Organic Chemistry II. 🍉🍉
Aww, thank you!
Any jee aspirant? 🇮🇳
I'm unsure of what you're asking. Let's stick to questions on the video!
@@Leah4sci i was asking peoples in the comments section, that if there are any jee aspirant who are watching your videos. Lemme explain, JEE is one of the most toughest examination in the world, many students (~1 million) in India🇮🇳 appears in it. I'm too a Jee aspirant. And your videos are helping alot, this concept was harder for me to understand from my teachers, but you made it clear to me, Thanks ma'am for your videos.
you>>>a Vanderbilt education
Wow, thanks!