Carbohydrates - cyclic structures and anomers | Chemical processes | MCAT | Khan Academy
Vložit
- čas přidán 6. 09. 2024
- Created by Ryan Scott Patton.
Watch the next lesson: www.khanacadem...
Missed the previous lesson? www.khanacadem...
MCAT on Khan Academy: Go ahead and practice some passage-based questions!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s MCAT channel: / @khanacademymcatprep
Subscribe to Khan Academy: www.youtube.co...
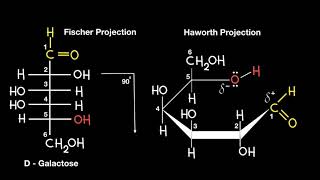








the way I remember the difference between alpha and beta glucose is that the letter alpha looks like a fish so the hydroxyl group is "swimming under the surface of the ring" and beta is like a bird so the hydroxyl group is "flying"...hope this helps someone else out!
+Timo Kuerten Be aware that alpha=down and beta=up does not always hold true. The anomeric OH relative to the CH2OH substituent on C6 determines alpha and beta designations. Alpha position is trans to CH2OH and Beta is cis to CH2OH. For example, the fructose unit of a sucrose disaccharide is the beta anomer, even though the anomeric oxygen is pointing down. This is because the anomeric OH is cis with the CH2OH substituent.
+Tobin Thuma fair enough my description is only what I learnt in my first year of Biochemistry from one of my lecturers so don't hold me to it
lol I just remember by "betta look up!"
My professor distinguishes the following way: alpha ants as in crawling on the floor so they are down, and beta bees as in flying up above. hope this helps
Beta anomer is the more stable form because in this setup most the large substituents such as OH and hidroxymethyl groups arange in a way that they are equatorial to the ring so they do not interfere with the stability of the ring structure. Keeping that in mind it makes sense that for the beta anomers the glicosidic OH group points the same way as the Hydroxymethyl (cis) group and for the alpha anomers they point in different directions (trans) Therefore your assumptions that alpha is under beta is above is wrong. It would be better to say that if the glycosidic OH group is opposite to the CH2OH group then it is an alpha anomer and if they are pointing the same way it is the beta anomer. I hope this helps:)
some (hopefully helpful) mnemonics for you guys:
Mnemonic: Sugar Daddy Loves Amino Acids
- D-sugars
- L-amino acids
Mnemonic: being lactose intolerant makes my Belly hurt
- lactose = glucose + galactose
- B in belly for beta glycosidic linkage
Mnemonic: Apple is a fruitose that has sugar
- sucrose = fructose + glucose
- A in apple for alpha glycosidic linkage
Emily Nhan thank you so much
Thanks dear
thank you!
Thank you! Wow that was really helpful. I am studying for the MCAT and that cleared up some questions that I have had for some time. Thanks again, I feel smarter!
The way you simply breakdown each step while completing a more complex concept really streamlines the learning process. I would love to see more examples or a practice section, where we could pause and complete and then check our accuracy.
I love the little learning tricks you give. You're awesome thank you. ^_^
iam sorry for being dumb but how does carbon 3 become OH upward when it is HO left on its fischer form???? 😅 and as well as carbon 4 become HO below when it is OH right?? i know that right is down and left is up but i just dont get the part with the OH and HO changes thingy and if it does matter if it changes,,, i really dk im dumb asf. i have test tomorrow and i'm still stuck at this
i need an answer huhuhuhuhuhu
Hey can anyone answer a question. Why in glucose, the number 5 carbon creates bond with number 1? What is the problem with carbon 4? Why not It's hydroxyl group reacting with the carbonyl group in number 1 carbon?
How does this have no comments? This video is very helpful.Thanks!
Beautiful explain.
thanks Ryan that was very helpful to me specially with lack of valid sources in my (persian)language
this is legit the best khan academy video out there... bless
I really appreciate the little memorization techniques!!! Thank you :)
This video is highly recommended..good work done Sir.
thanks!
What about the anomeric effect???
you rock man, those memory tricks really helped
It all makes sense now!! thank you so much!!
good video it help me a lot, i speak spanish as a first languaje and couldnt find good videos like this one
How do you know if for carbon # 1 the OH goes up or down? I don’t think you covered that
If the product is alpha, -OH will always be under. While beta always go above.
can someone explain why the OHs in the alpha D-sorbopyranose of the anomeric carbon and the highest stereocenter are actually on the SAME side (both down) as opposed to anti? And then conversely why the Beta form has the reverse
Thankyou so much!
dont we show thick n thin lines in the ring to show the closeness towards the reader?
i like that you said "peenk" at 1:57 LOL cus same
amazing tutorial
Owesome video! what kind of software you use to make this video?
Very helpful
موفق استاذ شرح أكثر من رائع
It was a fantastic job the way u've explained it . Thank you ^^
Thank you so much,this is very helpful 🌷
Thanks
helped me a lot
This video is awesome! Thank you!
most trusted channel :>
Very helpful! Thanks :)
wonderfull viedos
What if the glucose was L , will the anomers be L too?
When a compound changes into its ring shape here 2 hydrogen atom becomes less...like cycloalkane from alkane..
But in carbohydrates this method is not applicable why..
There both the structures have same number of hydrogen..🤔
3:30 saved me! thank you so much
May God bless you :))
A wise professor once told me alpha ankle (down) beta balloon (up)
sal must have a cold, he sounds funny.
I didn't understand the chair structure
Wrong Cyclic.
4:00 pyro for 5 carbones* and furo for 4 carbones
62:38
♡♡♡♡♡
I wonder why Khan Academy has so many subscribers....
guys..I hope you make arabic subtitle
Yes i hope to
Too complexed!!!! Make it simple
His accent was kind of distracting me. Lol Anyone else?
+Tasty Watermelon yup. looking for this comment