Titration of a strong acid with a strong base | Chemistry | Khan Academy
Vložit
- čas přidán 30. 08. 2014
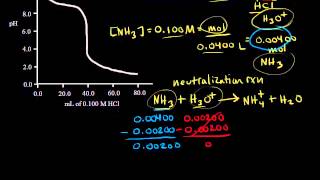
- Calculating the pH before the equivalence point for titration of strong acid, hydrochloric acid, with strong base, NaOH. Created by Jay.
Watch the next lesson: www.khanacademy.org/science/c...
Missed the previous lesson? www.khanacademy.org/science/c...
Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is the study of matter: its composition, properties, and reactivity. This material roughly covers a first-year high school or college course, and a good understanding of algebra is helpful.
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Chemistry channel: / channel
Subscribe to Khan Academy: czcams.com/users/subscription_...








i can pretend i understand it
plz mention end point, indicators, buffer region, nd equivalance point too
This video is perfect . Very well explained and right to the point . Easy to understand compared to my chem prof ...
Thank you very much it was exactly what i was looking for
very well explained sir ! thank you
that's the very first "not that helpful" "complex" khan academy video i've ever watched but anyway thanks for the effort
Thanks!
thank you so much sir
This man is the best. Thank youuuuuuuuuuuuuu
thank you!
Thank u so much sir
thank you soooooooooooooooooooooooooooooooooooooooooooooooooo much
Thanks for the video it is very useful:) I have an other (for me easier ) way to find the concentration of H3O+.
You have all in all 20 ml of HCl , it will be added 10 ml of NaOH which is 1/2 of the whole amount.
So you can say: [H3O+] = 0,5*1/2* 20ml/30ml = 0,167
PH = -log (0,167)= 0,777
hello just want to ask. Is the hydronium ion concentration of a strong acid titrated with a strong base equal to the concentration of the strong base at the equivalent point?
At end point/ eq.point conecentration of hydronium ion is more than conetration of base in that case that is why solution is acidic in nature because all base moles have been neutralized with moles of acid but acid moles are more than base
Lifehack: put it at the speed 1.5 and Khan Academy will speak like normal human 😄
Really? I feel like he speaks so much faster in the chem videos compared to the math ones
Is it possible to titrate a strong base (analyte) with a strong acid (titrant)??
Yes, the curve will be the opposite of this one, meaning that the starting pH will be high.
can the calculation of the titration of strong base and strong acid use the handerson hasselbalch eqn?
no. you can only use the henderson.hasselbalch equation for weak acids because every molecule of the acid is deprotonated. the equation would then be: ph= pks - log(0/A-)
-> ph = pks - log (0). log (0) is not defined
Artischoche k. Why did he use log to find pH? I can't understand how to find the pH. Could you please explain?
Nina Dobrev that's the formula for finding pH
No. The Henderson eq is used ONLY for buffers
anybody help me in solving some chemistry problems ASAP ??
how do i find Ka from this
i know i am 2 years late , but maybe its gonna be useful for someone else .
you cannot find the Ka because this is a strong acid and base solution .
@@benhardsim8629 After 1 years you helped someone with the same question.
pozdrawiam 8 grupe !
Thanks so much for the video, but I think all the extra explanation like converting ml to L is very excessive if this video was made for students in organic chemistry....
Skid some do this in high school so let it be Mr. Chemistry
no skid is right if you're doing this in high school and don't know how to convert moles and mL and all that crap you are the anomaly. The conversions are tedious
Skid I'min high school too and I think it's unnecessary
I didn't understood why the concentration of H3O+ was also 0.500M.
Strong acids completely dissociate in aq solutions. So, the concentration of H3O+ is going to equal whatever the concentration of HCl is because there is no base added yet.
In other words, every single H+ that was once bound to Cl- is now bound to H2O.
why cant you just get khan to explain ...
The MCAT can go to hell for making us do this without a calculator.
thank you so much sir
thank you so much sir