Introduction to Galvanic Cells & Voltaic Cells
Vložit
- čas přidán 15. 12. 2017
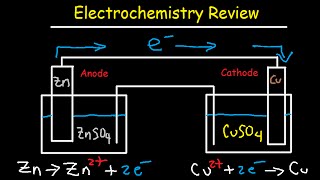
- This chemistry video tutorial provides a basic introduction into electrochemical cells such as galvanic cells also known as voltaic cells. A galvanic cell is device such as a battery which converts chemical energy into electrical energy. A galvanic is made using two electrodes known as the anode and the cathode. Oxidation occurs at the anode and reduction occurs in the cathode. The electrons are written on the right of an oxidation half-reaction and they are located on the left side of a reduction half-reaction. Oxidation involves the loss of electrons and reduction is associated with the gain of electrons. Electrons always flow from the anode to the cathode. The purpose of the salt bridge is to prevent the build up of charge. Cations flow through the salt bridge toward the cathode and anions flow toward the anode. This electrochemistry explains completely how a galvanic cell works. It discusses how to write the overall reaction from the half reactions and how to represent the cell using standard line notation or cell notation. The electromotive force of a galvanic cell is measured in volts and 1 volt is 1 joule per coulomb. Voltage represents the work that can be done per coulomb of electric charge. Electric current is the rate at which electric charge is transferred. This video discusses how to increase the voltage and current delivered by a galvanic cell.
Intro to Galvanic & Voltaic Cells:
• Introduction to Galvan...
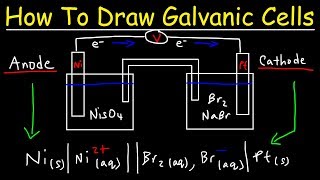
How To Draw Galvanic Cells:
• How To Draw Galvanic C...
Standard Reduction Potentials:
• Standard Reduction Pot...
Cell Potential Problems:
• Cell Potential Problem...
Cell Notation Problems:
• Cell Notation Practice...
___________________________________
Concentration Cells:
• Concentration Cells & ...
Cell Potential & Gibbs Free Energy:
• Cell Potential & Gibbs...
Cell Potential & Equilibrium K:
• Equilibrium Constant K...
Nernst Equation:
• Nernst Equation Explai...
Electrolysis of Water:
• Electrolysis of Water ...
_____________________________________
Electrolysis of Sodium Chloride:
• Electrolysis of Sodium...
Electrolysis & Electroplating Problems:
• Electrolysis & Electro...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
SAT Chemistry Subject Test Review:
• SAT Chemistry Subject ...
Carbon -14 Dating:
• Carbon 14 Dating Probl...
Beer Lambert's Law:
• Beer Lambert's Law, Ab...
______________________________________
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tutor.net/chemistry...


![Eminem - Houdini [Official Music Video]](http://i.ytimg.com/vi/22tVWwmTie8/mqdefault.jpg)




Next Video: czcams.com/video/vLCf4_NKjGU/video.html
Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Final Exams and Video Playlists: www.video-tutor.net/
The organic Chemistry Tutor is the hero this world needs
✅✅✅✅
Not the hero we need, but the hero we deserve
dude saved me from physics and chem. love him
He Batman.
@@sandeep7973 not the hero we deserve, but the hero we need.
He has a soothing voice.
He knows a lot of topics.
He does it for virtually free.
Thank you, sir.
he gets paid a shit ton from CZcams sooo that's def his incentive
@@johnnybones5406 but he could sell these materials and make just as much or more. He deserves all the respect
TOCT has been a hero of mine for many years.
@@seungseungminji He does sell his lessons lol. His full lessons are 2 hour+ long. The videos he posts are appetizers in comparison. Still very useful and just enough to get through college, though.
@@TactlessGuy I mean, it IS a business. My argument was, he does make money, which is okay. He could also not post these “appetizers”
Yo, why is he so underrated? He has been saving lives since idk forever.
In case someone hasn't already mentioned it, at 9:14 you mistakenly call the Zn 2+ an anion -- you correct yourself in the following discussion.
I was going crazy istg, thank you so much!!
Damn 15min of this video and understand more than i did after a full trimester of chemistry... thx👍
OC: You are so good! Please figure out a way to get heavily rewarded and further spread your incredible way of explaining things. YOU set the standard for teaching the sciences. No one is close!
Thank you for your excellent lessons.
And hello everyone!
wish you a good day.
I'm from Iraq and I'm learning maths and chemistry from this channel. My English is not very good, but I can understand the important part of each lesson. Our schools are very bad as you might know and the Arabic and Kurdish content on CZcams is poor in Science. So I have to come here sometimes.
Love for everyone from Iraq-Sinjar mountain
A salam alaikum
iraqis are here
@@waynekottkamp8591salamu alaikum wa rahmatullah akhi
I'm always amazed by how makes a topic so simple to understand.
your name amazing
bro taught me more in 5 mins than my chemistry teacher has in months
Me tooo😂
😂
I owe you my children, this video helped me so much! Thank you!
Hi dad
Nyrell Martinez Son, is the tutor treating you well? You haven’t sent a carrier pigeon in years
@@arnauldmartinez746 lol
@@arnauldmartinez746 dad im grown up now leave me alone
You are a literal genius. I am more grateful to you than you'll ever know. I owe you my deepest thanks!
I always use *Red Cat* to know that Reduction's at Cathode
You can use "an ox" as well!
@@jackshayne1634 yeah learned that a few days later lol
That's nice. Now I'm not going to forget it .
Oooh thank you
That only applies in galvanic cells right?
been staring at my chemistry book confused for days, watched the first ten minuted of this video and understood. thanks man, wonder how many people would fail chemistry without you.
Here's a tip from a fellow student to another, there is a good way to remember Oxidation = anode and Reduction = cathode. Using the phrase "an ox cared" You can know an=anode ox=oxidation and cared = ca and red which both respectively means cathode and reduction
Some books' got anode as positive and cathode as negative... it's so confusing 😕
@@mosespumpuni2631I think you might be getting it confused. Theres also a thing where electrons (negative charge) flow to cathode and positive charge flow to anode, which makes sense because anode is negative which attracts positive and vice versa. But the charge of the anode itself is always negative and cathode positive. Hope this helps.
@@mosespumpuni2631 That depends on the cell. Galvanic Cells Anodes are negatiive and positive cathodes. For electrolytic cells is flipped. The anodes are positive and cathodes are negatives.
Still regardless dont pay attention to that. Oxidation happens at anodes, and reduction happens at cathodes regardless of its the cell is galvanic or electrolytic. Thats the part to remember
Thats good. Another good one is Red Cat. Reduction happens at cathode.
I've been trying to learn electrochemistry for 6 months now. I thank God for you!!
I know you get millions of "thank you's" from people all over but you are AMAZING! Thank you so much!
We need that explanation of the chemistry in The Whole Arab World so badly....!
Finals week anybody? I'm about to binge this man's videos for the next week.
This explained the galvanic cell better than my Chem teacher explaining it for an entire week
i always watch the ads as a return in the favor lol
i dont know why this makes me laugh so hard
@@Celinekho05 bland 😒
YES YES
Simply watching ads wouldn't do.. click on the link in the ad, that's how you tubers earn.. they earn for every ad click
You're single handedly destroying Khan Academy, my friend. This is not a contest or anything. The more the merrier ... but you are the DEAN on on-line learning!! (Prof. Dave is a close 2nd).
You should run for president in 2024 dude. You're an absolute hero.
you are such a king, hope you have a wonderful day because you deserve it 🙏💗
You are honestly amazing, u teach me more in a whole day of ur videos than my chemistry teacher does in 9 months
Sir, I thank you to the ends and back, I refer to your videos right after classes and usually before them, these free lectures of yours meal my noggin better than the lectures given by IIT teachers whom i pay for. 😶 un believable. Hats off sire!
I'm Soo greatest to see your videos especially in chemistry,, ur explain things practically...thank you
If we can clap for the NHS then we can clap for this man..both are saving lives👏🏾
I don't know whether my teacher is making it complicated or you are making it simple.
I don't know how to thank you 🙏, I am so happy while watching your videos , since I am studying in easy way . and the way you explain. is understandable .THANKX💛
Thank you so much. You makes things so easy to understand and you cover everything. There's an explanation for everything that happens.
what you don't know is the word I don't Know... What don't you know?? Simply you are phenomenal....
This god of a human being helped me with Chem 12 and now for MCAT. Without him I would've been doomed
Today we were doing electrolysis and i asked my chem teacher about this and they said that it wasn't in the syllabus and that if they explained it it would just complicate the learning of the chapter.
Thanks for nourishing my curiosity✨
once again, you have save my life.
Its really amazing. I really liked your style of teaching. Please keep making the harder stuff easy for the beginners like us.
Here because I'm building earth batteries. Needed to recap on stuff I learned and forgot 20 years ago. The salt bridge isn't required in an earth battery because the soil does the same job :) Thank you.
Why nobody discusses the salt bridge in detail.
Dwift did
It’s used to prevent the mixing of the half reactions and balancing them out :)
To retain charge balance
@@lindddddactually its to retain the electron flow of the voltaic cell
@@mattheq9930that's the same thing
I am impressed: this is a very good video - clear and well told.👍👍
You teach everything wish I paid you vices my professors. Lol
Thank Gd for you!!!! You are the reason I get A's in chem
He deserves a teaching award
He deserves a nobel prize award...he's been saving students life since ages🎉...dude's a legend💥
Very good explanation! Thank you very much
He also has a soothing voice😊
I would’ve totally failed in ap chem if I had not known you sir sending u so much respect
Even my experienced chemistry teacher used this video for his lesson, you deserve RESPECT!
Thanks a lot, and have a great day every day!!
I wish this video came out sooner. Just had my final a couple days ago. Regardless, great video mate
Rizza same
I owe this video so much
My best tutor
جزاك الله خيرا
You are the smartest person to explain it in a simple way,,, you are the real academician bravo
Very good explanation.
You are the best chemistry sir ever 😜
I love you!!! thank youuuuu for your time and dedication.
thanks for the brief lesson
Well presented - Thank you
Thanks for this
It helped a lot 👍
Do the electrons travel only through the wire or will a small amount travel through the salt bridge?
Edit: I just found the answer on stack exchange. A small amount of current will leak through the electrolyte. To counter this modern batteries have a membrane called a separator that allows ion exchange but has a much higher resistance to combat the flow of electrons.
I owe this guy my life
No word for you are incredible
perfect explanation ❤️
Thanks a lot man❤
I can tell how successful this guy is by having to watch all these ads!
very nice,and clear concepts of the teacher. it's easily understandable to a commomn and verage minded person. This is greatest success of a good teacher
Hope to receive such type of lectures. S
Sir please explain cathodic , ICC protection of Marine ships to protect submesed part of the ships like Hull, Rudder, propeller, shaft ofthe main propelling engine,
You don't animate much but man You're better than Crash Course :)
thanks you very much teacher from somalia
You are the man❤
Thank u so much my superhuman friend
easy to understand👍
to remeamber it just use "AN-OX" and "CA-R"
It is the ions from the salt bridge which flow into the two 1/2 cells to maintain electroneutrality.
you are a legend sir
Thanx bro love you ❤️❤️❤️
Concerning the salt bridge, if the zinc ions flow to the cathode side, will the copper electrode now get coated by zinc metal?
No. The zinc ions will combine with the sulfate ions from the copper side and form a salt
I just have one question. Hypothetically, if we had a Mg and Zn simple cell (one beaker) in a dilute acid, would the electrons on Mg go to Zinc or would the Mg react and reduce the H+ ions in the acid instead. I am presuming Mg would directly reduce H+ because Zinc is more reactive than Hydrogen. Also there is a bigger difference in reactivity between H+ and Mg too. ???????
Thank youuuuuuuuuuu!
hello,is this how the lead acid battery terminals named or does it mean ,cathode is the positive terminal of a lead acid battery and the anode is the negative terminal? or does it mean anode is the positive terminal of the battery and the cathode is the negative terminal of the battery?
Deserve my subscription
this guy keeps saving me 😇
Great ❤️
This man be good❤💥😎
Thank you so much
So how do you build the salt bridge? And how do you stop the electrons from flowing through it instead of through your load?
Thanks sir ❗️♥️
Finally getting the hang of this topic 😸😂 thanks to JG
you just saved my life
Wow thanks so much
you should be blamed for making me fallen in love with chemistry. Now, how can I break up.
Thank you 😊
Legend of all seasons 🇿🇲😹 ZAMBIANS love you sir.
you are legit better than my teacher
Very fine
excuse me i’d like to make a correction at 9:13
aren’t cations positively charged ions? think, ca+ion (plus for positive).
He did say it wrong, but then a few seconds later correctly again.
Nice video
Thank you sir
I dont get the electric potential part 10:50 . If the zn soln has a potential 0.76 and cu soln has a potential of 0.34, then shouldn't the potential difference be the total voltage rather than adding it ?
The vid made everything very clear. thanks for that
How do we know to add the sulfate when it is not in the reaction?
can we take any other metal aq. solution instead of Zn(SO)4 solution in the 1st pot?
Thanks sir
You are my online education #coronavirus