Organic Chemistry Reaction Mechanism Pattern Examples
Vložit
- čas přidán 29. 09. 2017
- leah4sci.com/mechanism presents: Organic Chemistry Reaction Mechanism Patterns explained in 2 complete reactions.
Need help with Orgo? Download my free guide ’10 Secrets to Acing Organic Chemistry’ HERE: leah4sci.com/orgo-ebook/
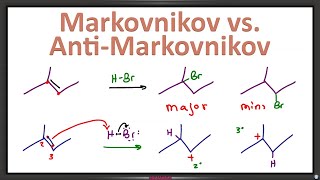
Every reaction mechanism is a series of arrow patterns that can be categorized as Nucleophilic Attack, Loss of Leaving Group, Proton Transfer or Rearrangement. This video walks you through 2 complete reactions showing you how to identify each step.
Reactions include SN1 reaction of HCl with an alcohol and the acid catalyzed formation of an ether from an alkene - same as acid catalyzed hydration but using a methanol solution.
Links & Resources Mentioned In This Video:
Video 1 and 2 in this series: leah4sci.com/mechanism
SN1 reaction (SN1/SN2/E1/E2 series) leah4sci.com/substitution-elim...
Carbocation Rearrangement Trick: leah4sci.com/carbocation-stabi...
Catch the entire Mechanism Video list in Organic Chemistry Series, on my website at leah4sci.com/mechanism
For more in-depth review including practice problems and explanations, check out my online membership site: leah4sci.com/join
For private online tutoring visit my website: leah4sci.com/organic-chemistry...
Finally, for questions and comments, find me on social media here:
Facebook: / leah4sci
Twitter: / leah4sci
Instagram: leah4sci
Pinterest: / leah4sci








It feels so good when everything that was confusing last semester suddenly starts to click and make so much sense!
Glad it helped you understand!
Degenerate: "Lord of the rings is the best trilogy!"
Me, an intellectual: Organic chemistry reaction mechanism part 1, 2, and 3 by Leah4sci
Hahaha thank you
Am i the only one who likes these videos, before watching and then, in the end feels proud of doing so.
I like these videos too ;)
You are a blessing to humanity, more grease to your elbow... You doing a great job. BIG THANKS LEAH4SCI
Thanks so much!
@@Leah4sci Always welcome
Thank youuuuuu so much for this, this is the first video I understand so much on this. Love youuuuuuuu ♥️
You're so welcome!
Thank you so much for all you do.
Nice work Team!
You're very welcome! Happy to hear you liked the video.
No adjectives can describe how good your videos are,
I have become a stationary group...
thanks so much! glad you're finding them helpful
Absolutely helpful! Thank you
Glad I could help :)
This helped me so much! Thank you!
You're so welcome!
You are super...! I have no words for you..!
Awww thanks so much!
OMGGGG, TYSMMMM, i couldnt even understand it before but u make it easier😭🫶
You are so very welcome! I'm happy that I cleared it up for you.
good explanation !
thank you
Very clear explanation Thank you
You are welcome!
This channel....is liiifeee!! I pray it gets a billion subscribes!♥️
Me too ;)
Very good instructions 👌
Glad you think so!
WOOW THANK YOU SOOOO MUCH I FINALLY UNDERSTAND GOD BLESS YOU
You're so welcome!
Very well presented! 😊 🎉
Glad you liked it!
Turning to be better than nuts now. Thanks Dear Ma'am
You're welcome!
If I'm correct, in previous videos, the 2-butinol + chloridric acid go to 2-chlorobutane and h3o+. But in this video it's water and not h3o+. Basicly I just want to know if it is a mistake from your part or does I miss something ?
It is water not H3O+. I checked other sources on the internet and the mechanism above shows why.
Mistake on my part, when reacting water + acid you get H3O+. Thanks for pointing it out
Very useful. Thank u.
You're welcome. Glad it helped you.
Could you please post videos of carboxylic acids , alcohols phenols and ethers ..
I cover these in the orgo study hall. Full details: leah4sci.com/join
Thanks
Ma'am plz tell how can we do mechanisms relating electronegativity and electron density...please
I'm sorry, I don't offer tutoring over CZcams.
"when oxygen yanked off the electrons binding it to carbon, IT LEFT"😂just kills me. you made it sound like oxygen was tired of the relationship
hahaha when trying to understand mechanisms, give the atoms human characteristics and you'll realize organic chemistry is more exciting than any soap opera
Can you please suggest a good book for organic reaction mechanism ?
Suggestions here: leah4sci.com/5-must-have-organic-chemistry-resources/
Hi,
Could you please post a video with examples of the stereochemistry?
already done! see the entire stereochemistry series here: leah4sci.com/chirality-stereochemistry-enantiomers-diastereomers-r-s-organic-chemistry-tutorial-series/
Thank you very much. Good job!
Is there any opportunity to stay in contact with you if I have got questions about stereochemistry? Because in 2 weeks I have to write an important test
yes: leah4sci.com/join
Thank you. Very helpful, what is the name of the formed ether?
At which specific point in the video?
@ 13:06
I cover this in my naming series. Be sure to watch the whole thing: leah4sci.com/naming
Being an 12th standard student preparing for KVPY and JEE, This is very complex form me!!!
(BTW I am from India if anyone don't know what these exams are)
Hope my resources are helping you reach your goals for your exams!
8:20 why does the pi Bond seeking for a positive partner attack the hydrogen which is only partially positive while oxygen has an actual postive formal charge?
to do that, you have to break the complete octet of oxygen and form H-. on the other hand, breaking the bonds to break hydrogen's octet and letting oxygen's complete is much more energrtically favourable. As a rule of thumb, you can remember that octet stability holds the higher regard than formal charges. Other opinions are welcomed!
@@ShauryaSingh-ts2oc I certainly agree with you. That is a good way to visualize it!
This is a great question! Even though it's oxygen that has a positive charge, the pi bond doesn't know it. huh?
When oxygen, an electronegative atom has a positive charge, it starts to pull on electron density from nearby atoms onto itself. By pulling on the bonding electrons between H and O towards itself, oxygen feels less positive. Meanwhile, hydrogen's positive nucleus gets exposed when the negative electrons are pulled away. In doing so, the pi bond only sees a partially positive hydrogen rather than a positive oxygen and attacks accordingly
at 1:21, why does oxygen attack the H+? Shouldn't H-Cl dissociate because it is a strong acid?
You are absolutely correct, in an aqueous solution HCl will completely dissociate to give H+ and Cl-, however in this case the solvent will be something less polar such as CCl4 and will not cause HCl to dissociate
you're a lifesaver ❤
Thanks so much!
Why oxygen atom in sulfuric acid is not grabbing the proton from methanol?
I'm sorry, but I don't offer tutoring over social media. If you ever find you need help with questions like this again, I recommend joining the organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
Omg, how helpful it is!
So glad to hear it!
The vidos are really good
I'm glad you think so!
Outstanding 😊
Thank you!
Where did you cover stability of carbonation?
here: leah4sci.com/carbocation-stability-and-ranking/
Concise explanation. 😊
Thank you!
Can Cl also attack first the hyrogen of OH
Cl- is not a strong enough base to initiate an attack on H+
In the reaction between H2SO4 and CH3OH .. why do the oxygen of Methanol attack first the hyrogen in Sulphuric acid? Can the oxygen of Sulphuric acid also attack first the Hyrogen of Methanol?
i have the same doubt .Moreover after the first step h2so4 became hso4-.so why did negatively charged nucleophile not attack
This is due to the present of CH3(EDG).
To make the OH present in the sulfuric acid a good leaving group
HSO4- is a very weak spectator ion and so dissolves in solution and does not react further. Oxygen on the solvent molecule is more reactive and that is why you'll see the methanol (or water or other alcohol) attack when the HSO4- does not
ma'am how do we know whether it will follow SN1 or SN2
see the substitution series linked in the description
Leah4sci ok ma'am and thnx
Superb🤓
thanks
at 6:21 here it is shown that the lone pair of electrons on the ch3oh pulls the h from the h2s04.
but why doesnt the lone pair of electrons on the h2so4 pull the h from the ch3oh?
The favorability of this reaction- where a proton is removed from H2SO4- is mainly due to sterics. Adding a methoxide to a molecule that already has four bonds around its central atom would create one very bulky (and unstable) molecule.
AH THANK YOU!!! THANKS SO MUCH FOR REPLY AS WELL. RELALY APPRECIATE IT
It's actually going but my issue is the carbon skeleton my teacher always use the CH3 instead of the line Carbon skeleton so it's a kind of confusing me
I'm sorry you're confused! For help with questions like this and more, I recommend joining the organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
what happens to the negative sulfuric acid?
It simply dissipates as a bored and non-reactive spectator ion in solution
Dissolves not dissipates
At 7:36, Why is there only one hydrogen transfer??
Are you referring to the deprotonation of H2SO4? If so there can be 2 as sulfuric acid is a strong acid. however, for the sake of this mechanism and a balanced reaction I chose to show just 1.
At 7:28 how is there a positive charge on CH3-OH2
OXYGEN is sharing 3 electrons and has 1 lone pair
Therefore,( number of valence electrons(6) - number of electrons present at atom(5)) it has a positive charge
Use the formal charge shortcut that I teach here so you can see where and how the charges appear leah4sci.com/formal-charge-formula-and-shortcut/
Nice
thank you
how did you just randomly bring in another methonal ??
It's not random. Remember that this entire reaction is happening in a methanol solution. There is an abundance of methanol molecules floating around in the reaction container. It's not just one isolated molecule.
Super
Thanks
What does the good leaving group do? ...... It's leaves😂😂😂😂😂🤣🤣🤣🤣
Glad I could give you a laugh :)
@@Leah4sci i deeply appreciate the way of your explanation thanks
@@Leah4sci ur leasons are really helpful
😀😀
:)