Reaction of Aluminum and Hydrochloric acid (concentrated)
Vložit
- čas přidán 16. 04. 2022
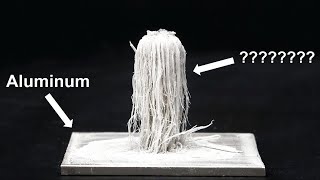
- A quick video showing what happens when Aluminum metal is put in concentrated Hydrochloric acid. In this single replacement reaction the Aluminum replaces the H in Cl. That results in Hydrogen gas (those are the bubbles) forming and aqueous Aluminum chloride.
The unbalanced equation for the reaction is:
Al (s) + HCl (aq) = AlCl3 (aq) + H2 (g)
Note that the reaction is exothermic and does get warm but that the water isn't boiling. What you see are bubbles of Hydrogen gas (H2).
The initial reaction shown at 0:00 happened much faster because the HCl (aq) was hot from earlier reactions I did. in the slower reaction the HCl (aq) was room temperature and took a while to get through the Al2O3 coating on the Al (s). But once through, it took place rapidly.
The white "fog" produced is actually water droplets from the H2 bubbles popping and from the heat of the reaction. While there is H2 gas present, it is not visible to the human eye.








Thank you for this video.... very easy to understand 🙏🏻
Even i had finished my secondary school, i still manage to watch videos from this channel every day
Thanks Dr. B, you're awesome
Thank you, that is most appreciated!
My chemistry is very weak but after coming your channel my chemistry become too good .... Thanks sir .... Love'from India 🇮🇳
That is great to hear!
Bro your english is also very weak 😂
Very good bro
Thanks!
It's really helpful 👍🏻💯...thanks sir....btw from India 🇮🇳💫
Thanks!
u helped with my chem thanks you
Amazing video 🤩
Glad you liked it!!
@@wbreslyn thanks sir 🙏🙏🙏
Amazing
Good vid man. I like the demos.
You have anything on Gibbs energy, spontenaety and a couple examples showing how to determine with given reactants?
Thanks! I've not done anything on Gibbs or thermodynamics at this point. Interesting stuff. I'll put it on my list!
@@wbreslyn Excellent, thanks man!
nice :)
Thanks!
What is the safest way to dispose of the aluminum chloride afterward?
How to turn off incandescent torch light in present of which chemical reaction
And today is my birthday
Happy birthday, Rumana! 🎂🎉
That is awesome! Happy Birthday!
Selamat Ulang Tahun in INA languange🎉
Kkk
Science is cool
So is Dr. B !
Thanks!
Mantap 👍
Hi,
Does this solution is uesd as Coagulant in Wastewater treatment.??
Hello, regarding this chemical reaction, I have two important questions. How should I ask the questions?
What's the colour of 2AlCl3(aq) solution?
Please make one on how to balance
KHCO3+H2SO4=K2SO4+CO2+H2O
Here you go, just for you!
czcams.com/video/epVOqTdcuzA/video.html
If you do more AL, is there a moment ( depending on the amount of AL ) you have liquid AL that can be used for modeling?
I'm pretty sure even if you pull it out its gonna be oxidised again after a minute
how concentrated it is, in percentage values? 30?
This is 37%. That's the most common percentage when when we're talking about conc HCl.
how i can synthesis poly aluminum chloride?
This is one way:
patents.google.com/patent/CN103738991A/en
wait im confused can u help me, where is the redox reaction here?
There would be redox since the oxidation states change on the Al and the H. But this video didn't go into redox.
Finding Oxidation Numbers: czcams.com/video/iSAwDJTLIKY/video.html
What is the reaction between the oxide layer and HCl?
I was wondering the same thing. I presume it's aluminium chloride and water but I'm not sure. Let me know if you found out
@@milosradakovic3 I found a study that said the Al2O3 reacts with H+ Ions that dissociate from the HCl. This is supposed to make Al3+ and H2O
2Al+6HCl--> 2AlCl3+ 3H2
❤️
Thanks!
Hi sir please help me sir
Which chemical apply on copper metal it defuse the filament 2.4 torch bulb can u tel me chemical name
You should consider making a Patreon so we can support you.
Thanks for the suggestion, I thought about doing that but haven't gotten around to it!
Sooo, I can safely eat aluminium then? Right? Right???
I suppose it depends how much!
Hey there,I had a quick question that is just floating in my mind,in the electrolysis of water we get h2 and o2 gases separated right.But then if we add salt for conductivity wouldn't it change the chemistry and result ? And if yes then what would change ?
Actually, if you had distillled water it would be difficult to electrolyze. You need some sort of electrolyte, like NaHCO3 or another soluble salt, for the current to flow. But if you were to electrolyze a saturated solution, of say NaCl, it would affect the products. See
chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.)/17%3A_Electrochemical_Cells/17.03%3A_Electrolysis_of_Brine
What is the concentration of the HCL?
(In molar please)
@@Heyyy102 About 12M.
Alright thanks!!
Please is this hydrogen chloride gas? Will the steam carry with it compounds of metals?
Nope, it is H2 gas. The bubbles and heat could result in some HCl gas. I suppose I could have held a piece of pH paper over the beaker to see if that was the case. But it don't believe it did. I also don't believe a significant amount of compounds would be in the steam although the H2 bubbles could cause some splashing of droplets that could contain dissolved AlCl3.
@@wbreslyn Greetings, does the reaction of hydrochloric acid with aluminum produce hydrogen chloride gas?
@@Chamlan17 Hydrogen chloride gas is HCl. This produces H2 gas.
@@wbreslyn thank you brother. Does sulfuric acid with whole food give hydrogen chloride gas?
you want make hcl is a gas combination of sulphuric acid with table salt
Gas HCL 🤗🤗
Passivation of aluminum
It's interesting how Chloride ions make reactions like these go faster by pitting the Al2CO3 coating. This is a video I did on a small channel with CuSO4 and Al. It is very slow without the addition of Cl- ions:
czcams.com/video/lgX-7h-mEYw/video.html
@@Paonporteur That would be interesting to see! I'm not sure I have any 100% pure haloacids, unfortunately.
Al+6HCl:AlCl3+3H2
What about 3 more Cl products?
chào quang
A chemistry G-D
Helllllllllllllllllllloo Dina!!!
Good night sir and what happens when we react HCl with NH⁴
If we react HCl + NH3 we get NH4Cl. Like this:
czcams.com/video/-VY3O6bD9j4/video.html
Oh, and good morning!