SN2 SN1 E1 E2 Reaction Mechanisms Made Easy!
Vložit
- čas přidán 30. 07. 2024
- This organic chemistry video tutorial provides a basic introduction into SN2, SN1, E1 and E2 reaction mechanisms. It provides a chart to determine which reaction mechanism will yield the major product given a particular alkyl halide or substrate. This video discusses the stereochemistry of some of the products as well as any carbocation rearrangements that may occur such as methyl shifts and hydride shifts. This video contains plenty of examples and practice problems.
Access The Full 1 Hour 34 Minute Video:
/ mathsciencetutor
Direct Link to The Full Video on Patreon:
bit.ly/3kDe8P2
Organic Chemistry PDF Worksheets:
www.video-tutor.net/orgo-chem...
SN1 SN2 E1 E2 - 4 Hour Test Review:
bit.ly/3Bt4ghw
_______________________________________
Join The CZcams Membership Program:
bit.ly/46xaQTR
Full 1 Hour 34 Minute Video on CZcams:
• SN2 SN1 E1 & E2 Reacti...







Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Full-Length Exams & PDF Worksheets: www.patreon.com/MathScienceTutor/collections
Access The Full 1 Hour 34 Minute Video: bit.ly/3kDe8P2
SN1 SN2 E1 E2 - 4 Hour Test Review: bit.ly/3Bt4ghw
It’s actually kind of sad that this guy can teach me in 40 minutes what takes my profs 3 hours… love the conciseness
Some people are natural teachers and most professors aren’t
paying someone who’s not even doing there job. it’s disappointing but that’s how life is now :(
Indeed he is good teacher. But you can learn from it because you have some basic knowledge about it.
The other 2 hr and 20min is explaining why all this shit happens. This is just a cheat sheet. Useful yes. But if you don’t know what E2 means, it’s useless.
@@Grak70 except in those two hours and 20 mins he ain't explaining shit. Just rambling on expecting it to click in our brains.
i miss when this was just nomenclature
I have an orgo chem test in 2 days, and I work a full 8hr shift in between, this is an INVALUABLE review session!!!
How'd your test go?
@@PunmasterSTP I got a 76 for the final (which was WAY better than what I was expecting) and I passed the class _with a B!!_ 😊😊😊 not a terribly high one, but I wouldn’t have been able to even have half a shot at passing without his videos!
@@norainnoflowers1551 I'm glad to hear that!
And I'm glad to read these comments 😃
It is one of the greatest gifts we have in our modern technology era to be able to teach millions (probably closer to billions at this point) a variety of subjects with the level of mastery you possess... and for the most, part free of charge. You are simply the best.
i appreciate all your work and effort into these videos. I tend to fall behind a lot in classes, so this is great for me to study with. Very much appreciated. Hope you see this. Keep uploading, your helping people all around the world. ♥️ty.
Wow I can't believe how time flies. I remember watching the organic chemistry tutor for algebra II in highschool and now I'm actually taking organic chemistry and still watching. you are the #1 GOAT channel
This is the most helpful video I've ever watched so far. We can't thank you enough man. Our professor took him a month to teach us those and you just taught us those mechanisms in 38 minutes
Me who's not even taking organic chemistry:
I like your funny words, magic man.
Me, except I am taking organic chemistry… for the second time.
SN2 SN1 E1 E2? More like "Super great amazing lectures for you!" The chart as well as the entire rest of the video was solid gold, and judging by the comments, you've already helped countless people, myself included.
This was amazingly well done! I could follow along easy when I've been struggling to find material to learn these reactions with.
Ya I'm convinced I owe you all of my tuition. You taught me more in this 38 minute video than my prof did in 1.5 months
Thank you so much for this. I needed some refresher courses for a standardized test I may decide to sit for.
Hey I was just curious. Did you decide to take that standardized test?
One of your best video's imo. Not the easiest subject, but you explain it so clearly! I'm half way of the first year of my bachelor, and for literally every subject I've had so far your video's came in handy. Thank you so much!
Thank you for the videos. You save lives Man. Thx
This video is very useful to me , thanks a lot.
I was just about to try and make a chart like this as a reference to look back to while watching his other videos!
don't stop . you have made more video in organic chemistry... it is so useful for fundamendal learners.
😆😆😄😄😄😄
Thank you i have an exam today so this couldn't have come at a better time! God bless you.
I know it's been a year, but how'd your exam go?
@@PunmasterSTP i know it's been 8 months, but how'd your exam go?
@@buqbooQ I wasn’t actually in a class, I just tutor some people in various subjects and come on educational channels to brush up on stuff. But I do remember my ochem tests back in college and they were fairly tough.
Right on time, thank u so much
let me not lie to you man, its day before my exam and you have literally carried me through organic chemistry fr
Thank you for your explanation on this subject. It is very helpful
Why are you so much better than my chem prof
Thank you I've been waiting for this lesson
Thank you teacher from ethiopia 🇪🇹🇪🇹🇪🇹
Best video ever on This mechanism
The best video I have seen on this topic
I passed my midterm because of this video, Thank you sir!
😭😭you've just saved my soul.....thank you soo much
Just what I needed! thank you
literally the ONLY video that made me understand. Thank you!!
Very helpful 🥺❣️ love it
If anyone is confused about 16:09 and why the CH3OH isn't mentioned or why the SN1 or E1 reaction will not take place, he actually explains it in the next example at 19:45.
I have a test on this Monday! GOD BLESS AHAHA
LOL same girl Ohio State?
@@saniahussain6074 Go bucks! Good luck
WHAT A BEAUTY THIS IS !!!!!
like I could not understand my professor said at all, but now I am getting all the answers after watching a couple of his videos. Now I am thinking... there are three options. 1. I am dumb, but this dude is amazing in teaching concepts. 2. I am not dumb and this dude is amazing. 3. I am not dumb and my prof sucks and this dude is great. I think it's three. Hopefully lol
Thank you!!!!!! 🤩
shoot this is what i need to understand but do not know what is going on
why pay for tuition when I have you. life saver
On jah bruh you ain't mess around with these videos b
damn usually your videos really help me but this one confused me even more lol
im a huge fan ...any fun livestreams :') ?
YOU NEVER MISS
Clutch as always!
Thank you!
What a baller. Respect 🙏
WOW, zoom students everywhere thank you
Thanks admin
Bruh I needed this a week ago
ur the goat fr, thank you!!!
Just in time for my exam next week!
just in time for my exam tomorrow!
Good luck!!!
@@-snazzysnek-5570 Hey how'd your exam go?
@@justingenco7413 How'd the exam turn out?
@@PunmasterSTP I don’t remember lol but I’d like to think this helped for sure! In biochem this semester
this guy has saved every engineer's life
and pharmacists'
Absolutely
And entomologists'
I have to give my ACS exam today for organic chemistry 1 wish me luck!
Why he didn’t give the protic and aprotic solvents a value role in his answers ??
Wow I just learn this few days ago but I still don't understand who knows u suddenly upload bout this tutorial video.. NICE😂😂
thanks
Thanks!
Primary halide, it undergoes SN2. If use bulky base, the reaction goes in E2 mechanism. Incase of strong base it will proceed as an SN2 and if primary substrate is sterically hindered it undergoes E2 reactions.
summarization coming in clutch
Education attaches all continent 😊
Hello, i have an exam in two days, but I dont understand why in minute 34:50, it is an SN2 reaction. Because the solvent (CH3OH) is not appropriate since is polar protic. Why is SN2??
Which mechanism is in the first example ?
Thank you so much
Man I love you
Great
thankyou
Can you please clarify on example of the 2-bromo-3-methyl butane with the -OCH3. The solvent listed is protic, so would that not solvate the base/nucleophile and push the reaction toward an SN1/E1 reaction instead of SN2/E2?
-OCH3 is a strong base, so you would not be able to form C+
Kinda late but for whoever else was wondering, looking at problems like these I believe that even in the presence of protic solvent, as long as you have a strong base it will go E2/SN2. The solvent is a rule to follow, but more of a hint that MAYBE the mechanism will want to go E1/SN1, you have to look at the whole mechanism as a hole and figure out what will really happen. Usually most mechanisms can be dictated by looking at possible carbocations and the strengh/bulkiness of the base/nucleophile
Edit: Think of it like this, the NaOCH3 used will ionize into Na+ and OCH3-, it will continuously create another OCH3 by taking the H from CH3OH, therefore regardless of having a protic solvent, you’ll always also have strong base/nucleophile present. Now, here’s a tricky part, at the end then which will you get most of SN2 or E2? For this you have to look at the SIZE of the base/nucleophile.
Anything smaller than ETHANOL will act as a base = Elimination product
Anything larger than ETHANOL will act as a nucleophile = substitution product
WITH THE EXCEPTION OF: Acetate ion, Sulfurs, and negative carbons!
@@georgieacero7043 Hi, thanks for the explanation, may I please ask what you mean by "smaller" or "larger" than ethanol? Atomic mass?
@@user-jk8iu8io8o it’s a pleasure! And I simply mean the size when drawn on paper, it’s a simple trick that works
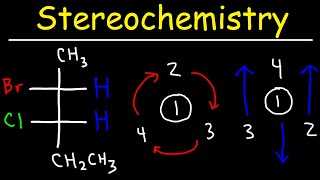
At 10:10 you say that the product is a racemic mixture and that the isomers could be wedge or dash, but is it possible for the OCH3 to be in the plane of the molecule--neither wedge nor dash?
Idk if this is too late but no it’s not possible because the stereochemistry of the leaving group it’s taking the place of is not in the plane itself. Depending on where the OCH3 comes from depends on which isomer form it becomes!
Tertiary alkyl halide favour SN1 mechanism and E1 reactions. Using strong base with tertiary alkyl halide favors E2 reactions.
16:54 for this example, Sn2 would not occur because methanol is a protic solvent, and Sn2 can only perform in aprotic solvents
Yeah that part confused me it’s in a protic solvent….
Fair point- I think that was just a slip-up on his part
As far as I can tell, he's basically trying to say that the base always takes precedence over the solvent. That said, yes. SN2 only occurs in protic solvents. He should have put a protic solvent there, so just act like there is. Pretty sure he just made a mistake- hope this helps
@@saadinhalf SN2 occurs in aprotic solvents not protic solvents.
The substrate is on secondary carbon and has a stronger base in the reaction, so even though the protic solvet is used, the SN2/E2 would occur. If it is a protic solvent but no strong base, then SN1/E1 would then occur. If you look at his table at the beginning of the video he lists the reactions depending on the solvents/bases used.
he also did this for the following problem with the sterically hindered base. usually hes good but this is kinda throwing me off
On the first example, why is it only the SN1 reaction that occurs and not the E1 as well?
Hi I would like to ask if my substrate is tertiary alkyl halide ( it is 3 hexane connected together and then attach to the carbocation carbon we 2 more methyl which gives u tert) with tert butoxide and THF what will be the mechanism?
i meant 3 cyclohexane
Great Sir
How is this an introduction? I need to know....
this is for free is a blessing
Secondary alkyl halide, if we use aprotic solvent like DMF, DMSO, I, CN it favors SN2 mechanism. If we use protic solvent Including water, methanol, it will favour SN1 and E1 mechanism. Sterically hindered Secondary alkyl halide with strong base and bulky group give E2 mechanism over SN2.
Father, thank you.
In one of your examples reacting 2-bromo-3-methyl butane with sodium methoxide and methanol you don’t do a hydride shift from the 3’ to 2’ carbon. Why not?
At 12:37, why would the Hydrogen be removed rather than the methyl group? why would it not form an alcohol?
28:40 since acetate is a bulky base, why would that not be E2?
thanks dad
im confused for his e1 products he doesnt draw the double bond
18:50
But you said that 2 degree substrates with protic solvents show SN1&2 and E1
reactions
Then why does this show E2 reaction and doesn't even show E1?
In case of methyl bromide, the reaction will proceed using SN2 mechanism. It doesn't matter what type of solvent is used.
At 16:21, why is it SN2 when there is a polar protic solvent ( methanol) and not SN1
Good
0:00 does substrate also mean electrophile in this case?
yes the substrate is the electrophile
Can someone explain why he was able to use another water molecule to remove a hydrogen atom in the SN1 reaction at 7:59 ... when the reaction only started with one water molecule?? I'm confused
Could someone explain what sterically hindered means
Sterically hindered basically means the reaction can't happen coz group is too bulky, in a way it literally body blocks the incoming nucleophile if the compound has multiple branches that's why tertiary C is bad for Sn2
It's like heavy groups surrounding an atom. As you can think of a carbon atom surrounded by 3 methyl groups which doesn't allow other molecules to attack on it. It basically gives repulsions to the attacking molecule.
In minute like 14:46 shouldn’t that “minor” product be the major one since the bad and carbocation are very sterically hindered or am I confusing something
@ 30:32 why is it an SN1 reaction?
Hello! At 16:45, could the C+ rearrange to a terciary and react throught E1 SN1?
that's what I was thinking, just reached out with this exact question to my prof. and we will see how she responds!!
8:36 why do we add that H next to the carbocation
Since its an elimination reaction, the water attacks a beta hydrogen, not the carbon, so we draw the hydrogen so we can visualize it
How do you know that methoxide is a strong base? Like what makes it a strong base?
It has a pKa of 16, same as OH. So it’s equally basic. Same goes for any -OR, as far as I’ve learned
You know that normally OH- is basic as it was the case for an acid like NaOH kOH, BaOH CaOH, just with OH- itself they are strong bases. Now if we replace H with an R group( electron donating), it will cause increase in charge over the oxygen which makes it more electron dense than OH- and hence a stronger base (think of it as a lewis base) than OH-, and OH- itself is a strong base (pOH~ 12-13)
At least this is a more logical way to think of it. I might not be considering somethings but anyway
How about allylic, benzylic, vinyl?
i understood this less and less as the video went on
What’s a bulky base?
I’m not sure because I’m not an English native speaker. But I guess it is a base that is very voluminous, so like it has a lot of large groups around it and acts as very bad nucleophile because of steric interactions.
An exemple would be : ethanolate is not a bulky base but the base of 2-methylpropan-2-ol is a bulky base thanks to the 3 methyl around the carbon linked to the negative O.
Sorry for my English btw, I’ve tried my best haha
Bulky base is something which has CH3 ( methyl group ) giving so much of steric hindrance to the molecule. Like , sodium tetra butaoxide. It's structure kinda looks like three methyl groups attached to the carbon atom which indeed is giving the overall molecule much hindrance.
watching this 12 minutes before my final 👍
this doesn't make any sense to me :(
Dumb
😂
i should have just started with this video instead of sitting through 2.5 hours of sub/elim lecture