Synthesis of aspirin
Vložit
- čas přidán 11. 06. 2024
- Aspirin is synthesized from salicylic acid and acetic anhydride using concentrated sulfuric acid as the catalyst. The purity of the synthesized aspirin is measured using the ferric chloride test as well as obtaining an FTIR spectrum.
The following lab techniques are demonstrated:
Using a warm water bath
Scratching glass to induce crystallization
Vacuum filtration
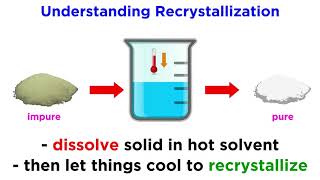
Recrystallization
Ferric chloride (FeCl3) test for phenol
FT-IR analysis








That scratch sound is so lovely 😂 lol
Do you have to use the Acid Catalyst if given more time? Have subbed good information
I enjoyed this thank you for the awesome content!
Glad you enjoyed it!
Hello! Can you upload the result of the FITR analysis? It would be a great help. Thank you.
Hey I start working in pharmacutical company in my country your videos help me a lot thank you very much
Congratulations!! 🎊🎈🎉
Indian student here ... enjoyed ... greatly done
Industrial scale, may I know the temperature at which the reaction is carried out?? Please reply ASAP
لماذا أضفنا الماء المثلج؟
Professor Smith, thank you! Can we skip the water bath and put it directly on the hot plate?
It depends on how much water or aqueous solvent you have in the flask. If there is only a small amount of aqueous substance in the flask, putting it directly on the hot plate may cause the reactants to heat up too fast and decompose. This is why a water bath is used for this particular synthesis.
If there is a lot of aqueous solvent, the high heat capacity of water makes it safe to put the flask directly on the hot plate without worrying about decomposition of your reactants.
@@RoxiHulet I see! You explained it so well. Thank you so much, have a nice day! 💖
why doesnt aspirin dissolve completey in water like the medicine does? wouldthe acetic anhydride react with the water and form acetic acid?
Aspirin isn’t very soluble in water. You can only dissolve about 0.3 g aspirin in 100 mL of water. When you dissolve an aspirin tablet in water, what you observe is mostly just the binder material dissolving, not the actual aspirin molecule.
And yes, acetic anhydride will form acetic acid when combined with water. I don’t use water in this reaction.
what is the importance or the role of magantic stiler and magnetic bar ?
To rotote a mixture?)
Pourquoi ajoute-t-on de l'cau
Organic Chemistry 2B. Sierra College Rocklin California
Today's my viva and I'm very tensed😔😭 wish me luck
how did it go??
This gave me a headache.
Hii Mam