Video není dostupné.
Omlouváme se.
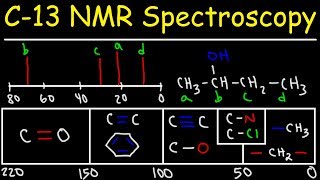
An Animated Lesson on NMR Spectroscopy
Komentáře • 23
Další v pořadí
Automatické přehrávání
An Animated Lesson on ChromatographyChemistorian
zhlédnutí 1,9K
Carbon-13 NMR SpectroscopyThe Organic Chemistry Tutor
zhlédnutí 497K
Will This Revolutionize Chemistry? (Organic Electrochemistry)Chemiolis
zhlédnutí 111K
WORLD'S SHORTEST WOMANStokes Twins
zhlédnutí 97M
Growing An Ear In Your Arm 😨Zack D. Films
zhlédnutí 68M
Crossing the Most Dangerous CrosswalkZach King
zhlédnutí 15M
My life changed when I met her❤️🥰@isabellaafro #love #couple #travel #shortsJaymondy
zhlédnutí 18M
Introduction to the NMR SpectroscopyOrganic Chemistry with Victor
zhlédnutí 1,9K
The Mystery of SpinorsRichard Behiel
zhlédnutí 854K
How MRI Works - Part 1 - NMR BasicsthePIRL
zhlédnutí 529K
A Chemist Explains the ENTIRE History of Atomic Theory (in 48 Minutes)Chemistorian
zhlédnutí 131K
The Origin of the ElementsJefferson Lab
zhlédnutí 2,6M
The FASCINATING 200-Year History of BenzeneChemistorian
zhlédnutí 129K
Attosecond Lasers (2023 Nobel Prize in Physics) - Sixty SymbolsSixty Symbols
zhlédnutí 426K
Investigating the Periodic Table with Experiments - with Peter WothersThe Royal Institution
zhlédnutí 1,1M
An Animated Lesson on Mass SpectrometryChemistorian
zhlédnutí 4,5K
Handstand train challenge!!The Rybka Twins
zhlédnutí 6M
A teacher captured the cutest moment at the nursery #shortsFabiosa Stories
zhlédnutí 51M
The way he played it off at the end 😂 (via colten1019/TT) #shortsSportsCenter NEXT
zhlédnutí 13M
Ariška miluje Tary Camp! 🥰🤩Tary
zhlédnutí 170K
TO NEJLEPŠÍ Z INTERNETU! #54 😍HouseBox
zhlédnutí 535K
Little girl's dream of a giant teddy bear is about to come true #shortsFabiosa Animated
zhlédnutí 9M
CHURAQ CLIQUE - STRÁŽEMilujeme Párno
zhlédnutí 140K
Every girls worst nightmare 💅😫 #anwar #hannahstocking #shortsHannah Stocking
zhlédnutí 6M








Hi everyone, I'm excited to announce that I now have posters available for the reaction schemes in this video:
Aliphatic reactions: rdbl.co/47y5oAk
Benzene reactions: rdbl.co/41W1qQM
Phenol reactions: rdbl.co/3NZc8QE
Perfect for learning organic chemistry (and supporting the channel at the same time!) 👨🔬
I can confidently say that you’ve saved my grade, thank you!
Great Video! I will sent it to other Students who Need help With NMR. Your explanation is very good and understandable !
I really wanted to thank you for this clear and concise explanation. I have my O-chem exam in a couple of days and for the life of me i couldn't figure out how to piece together a molecoule from a specter and vice versa. I knew all the teoretical bits about stretching and HOMO and LUMO but this practical part really puzzled me. Thank you for your part in educating the future chemists of the world and keep up the good work!
Best Explanation ever had
I get your recommendation from Reddit and absolutely loved your Videos
Incredibly well executed video! Thanks a bunch:)
Best explanation out there!!! Thank you endlessly
Thank you for this. Really informative!
Excellent video. Thanks to it I finally understand NMR. :)
Great to hear! Once you understand, looking at spectra can be kind of fun (in a very nerdy way). It's like solving a puzzle 🤓
At 17:36, could you please explain why the multiplet is a septet. Shouldn't it split to 9 peaks?
Great question and you're absolutely right. Well spotted! It's only like that because I was drawing these spectra manually, and they're quite fiddly to draw, but if I had more artistic ability, it would be split into 9 haha
@@Chemistorian don't mind it !! I appreciate your efforts!! Your channel is superb
Thanks for saying that, I'm happy to have helped!
hi, I have a question for the proton nmr at 13.02 how is it split into 4 environments when none of them are in identical environments?
It's quite hard to spot, but the two CH3 groups on the left hand side of the molecule are equivalent to each other. They're both CH3 groups coming from the same carbon atom, making them identical environments.
That gives us 6 protons all in the same environment, which is why the peak on the spectrum for that environment has an intensity of 6.
Thank you so much for replying
um I was actually asking about the beginning part of proton nmr where you said just like 13c nmr we can identify that there are 4 environments my question is how is it 4 environments? Is the molecule not an ester. I am kind of confused about which part of the molecule is an identical environment @@Chemistorian
The spectrum that shows at 13:02 is for isobutyl acetate. If you keep watching, I explain how to assign all the peaks in that spectrum. You can skip to 19:05 to see the final structure once I've gone through all the peaks, and I highlight the protons which are in identical environments to each other. Hopefully you'll be able to see that there are only 4 proton environments for this molecule.
thank you so much! i u nderstand now @@Chemistorian
I'm late to the party, but for the last problem, wouldn't a cyclopropanone with a methyl group and ethyl ether on one carbon also work? 6 carbons, 10 hydrogens, 2 oxygens, and the NMR matches, unless I'm wrong and the first singlet would be more shielded than a regular ketone due to the inductive effect of the ether group?
Why nobody can make a simple software to figure it out?
With your non-rhotic accent, "NMR" sounds like "enema". lol