Basic Concepts of Thermodynamics (Animation)
Vložit
- čas přidán 4. 07. 2024
- #thermodynamicschemistry #animatedchemistry #kineticschool
Basic Concepts of Thermodynamics (Animation)
Chapters:
0:00 Kinetic school's intro
0:17 Definition of Thermodynamics
0:52 Thermodynamics terms
1:40 Types of System
2:40 Homogenous and Heterogenous System
3:55 Thermodynamic Properties
6:30 State of a System
8:10 State Function
9:25 Path Function
Thermodynamics:
The word "thermodynamics" was derived from the Greek words:
"Thermé" which means “Heat” and "Dynamics" means “force”.
So, "Thermodynamics" is essentially the study of forces due to heat or heat due to forces.
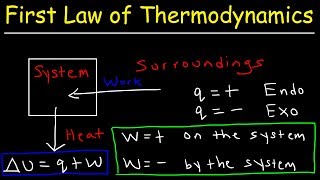
THERMODYNAMIC TERMS:
System:
A thermodynamic system (simply "system") is that part of the universe which is under thermodynamic study.
Surroundings
The space outside the thermodynamic system is known as the surroundings.
System Boundary:
Anything which is separating the system from the surroundings, is called the boundary.
Thermodynamics systems
can be described in three different ways:
Open,
Closed
And isolated.
THERMODYNAMICS Properties:
Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system's state.
In general, thermodynamic properties can be divided into two general classes:
Intensive properties and
Extensive properties.
State Variable:
When the properties of a system define the state of that system
is called State Variables.
State function:
A State function is a thermodynamic property of a system, whose value depends
only on the present state of the system and is independent of the path by which
that state was reached.
Path function:
A Path function is a thermodynamic property of the system, whose value depends upon the path by which the system changes from its initial to final states.







I love this video. It’s the only thing on the internet that really explains what thermodynamics is about and really explains its power
Agree❤
This was so helpful thank you ❤❤
Really great video! It explained the concepts very well and explained the very basics ❤❤ Now I am confident to walk into the first class of my thermodynamic chemistry.
Really helpful.
Appreciate you for that.
a very great explanation. pls keep makin further....
Great video information 👍 thank you for sharing your knowledge ♥️🇵🇭🫡
I'm a newbie to physics. Thank you for the concise explanation! The visuals are a great aid.
Glad it was helpful!
It is chemistry
Yaa in physics also is their thermodynamics
I really enjoyed the lesson, well done 👏👏👏
Very good animated video..👌
Very helpful
Well done
Wonderful
Best explanation 😃
Good Explanation...
IT IS GOOD EXPIANATION THANK FOR SHARING
very nice
Please make more videos on physics
Mam app kis app se lecture bnati Hn ????
thanks for the contact but you should add some more definitions its the basic and and the key for each quiz in thermodynamics and will help in heat transfer.
Tomorrow my exaam mam this very much helpful to me thank you mam
Wow
Explanation on energy
Work is a path function.. But it was not made clear through some more examples
Mixture of liquids not homogeneous
Ex. Mixture of oil and water
here we go again