Dalton's Law of Partial Pressure Problems & Examples - Chemistry
Vložit
- čas přidán 26. 10. 2016
- This chemistry video tutorial explains the concept of dalton's law of partial pressure. It provides the equations plus plenty of examples and practice problems for you to work on.
Pressure & Boiling Point: • Introduction to Pressu...
Gas Pressure Unit Conversion:
• Gas Pressure Unit Conv...
Manometers & Barometers:
• Manometer Pressure Pro...
Water Height & Mercury Column:
• Height of Water in a B...
Boyle's Law Practice Problems:
• Boyle's Law Practice P...
_________________________________
How Does a Bike Pump Work?
• How Does a Bike Pump W...
Charles Law:
• Charles' Law
Gay Lussac's Law:
• Gay Lussac's Law Pract...
Avogadro's Law:
• Avogadro's law Practic...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
Combined Gas Law Problems:
• Combined Gas Law Problems
_______________________________
Gas Stoichiometry Problems:
• Gas Stoichiometry Prob...
Molar Mass of a Gas at STP:
• Molar Mass of a Gas at...
Gas Density at STP:
• Gas Density and Molar ...
Dalton's Law of Partial Pressure:
• Dalton's Law of Partia...
Collecting Gas Over Water:
• Collecting Gas Over Wa...
_______________________________
Gas Density of Mixtures:
• Gas Density & Average ...
Average Kinetic Energy of a Gas:
• Average Kinetic Energy...
Graham's Law of Effusion:
• Graham's Law of Effusion
Kinetic Molecular Theory of Gases:
• Kinetic Molecular Theo...
Gas Law Problems Review:
• Gas Law Problems Combi...
_________________________________
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections








Final Exams and Video Playlists: www.video-tutor.net/
Bro You Are Him Like Fr Man You Are Him 🥲❤🔥
U do not just make my chem modules seems easier, youre also saving my mental health. I've always been a sucker when it comes to mathematics because I'm more into words and literature so whenever I'm commiting mathematical problems I'll always doubt myself solving problems because I think it's always wrong and then It'll turn out to be a mini-breakdowns and it makes me very anxious and I'll think that I'm such a loser in numbers and I'm stupid.
are you gay
Lol that's me most of the time ⏲, how I wish my perspective can change😪
@@mantuli_ka1 for starters y'all literally cant be dumb cuz A: most dumb people think they're smart. B: dumb people often don't try and improve/ better educate themselves. So its clear you guys are NOT dumb. Fortunately confidence is really the last thing that needs to be worked on( better you're solid at what you do then work on confidence than have it be the other way around). Fortunately building confidence is EASY! The more you practice the better your confidence in whatever it is you're doing (if you practice something so much to the point youre getting bored because it has become that easy its hard not to be confident in your skills - picture something as mundane as walking or even something like breathing [yeah you may choke every once in a while or get breathless but this happens to literally everyone not matter how good they are at what they do]. So Its not that y'all lack the smarts. its just the confidence. Y'all got this!
I was at this stage quite a long time ago.
Dedicating some pretty long months to math made me feel comfortable to it.
I'm good in numbers and mathematics but my grammar was really bad, I think we have different skills. But yeah I'm trying to fix my grammar. I'm having a hard time passing an english exams because it was confusing and idk bro. But fun fact. I passed in chemistry and failed in english 💀. Im 8th grader hehe
This guy makes skipping chem classes easy! my professor is amazing don't get me wrong, but i can't record his lectures to go back and study them again neither do i like to go to school when it's TOO COLD to get off my bed. i've made 95/100 and 92/100 watching videos on this channel. THANK YOU is not enough!
Saaaaammmmeeee
Must be nice 😭
I guess im failin chemistry 10 then.
Update i passed ❤️😂😂
Eeeyy great
Ayoooooo
@@GoodGuyCzy You giving me hope xD
@@spacecat3738 aye man if I did it you can too!!
these videos are so short but make the concept so easy, i found it beautiful for all revision work before my tests, super helpful! :)
I wish my Chem teacher taught like this!! I LOVE your videos! Thank you!!
Thank you very much. Here, In my country we are not used to online classes so it was difficult for us to understand what our teachers were saying but this video helped me a lot.....
thank you for all your videos! they're great to study with!
Definitely the best professor in every subject he teaches. You are more than awesome
another incredible video, you never fail to explain things better than nay textbook or professor could. Thank you for everything you do!
saved me in math...now in chem
Totally agreed
Like a super hero😅😅
Realll 😭
Wow! So clear and simple! Awesome video; thanks!
I've always wanted a chemistry lesson from Mark Wahlberg!
💀
Lmao damn
Eh?
Is this a joke? I have seen it a lot on JG video's comments but I don't get it, mind elaborating?
He doesn’t sound like him AT ALL
He says "thanks for watching" as if we did him a favor doing so..doesn't know he's doing us a favor uploading this for us, SAVING OUR ASS'S ☠️😫
My Ap chemistry teacher is pretty clueless so this tutor guy is saving my a$$
Dalton's a GOAT you know, man did all that himself oh yeah
Great video ! Thank you !
I love watching your videos.Thanks 🙏🏽
Thank you so much sir it was so helpful !
You earned another subscriber 😊 Thanks man
Welcome
As always, you are a god send. Thank you for what you do.
i got distracted by how you said 40 so cutely, "fowdie"
This is really helpful considering my chemistry teacher just made us play Minecraft and dropped a major fuck you on our finals exams with lessons we don’t know
Imma give my thanks to Organic chemistry tutor! I watched this video and then got 4 mistakes in my test! 🎉
that was easy to learn after some practice. I used to think it was really hard and i procrastinated learning partial pressures.
Thank you!!!
To this day your vids come in handy my guy
Thank you!!
With the video it really easy for me to understand. It help me alot
Thank you for a life saver video 👍
Love you~~~~~~~~~You are a great teacher!!!
I dislike listening to our science class since our teacher seems to be lacking with chemistry that's why I feel like "i ain't listening to someone who does not feel the lesson" and then ill skip classes bUT i never worry about the consequences because i have this channel skskskksksksksks ur my savior
when my profs recorded lectures are poop because of upload speeds. suffice to say I'd be well screwed without this guy!!!. A million thank you's!!!!!!!!!!!!!!!
thank youuuuuu so much you are the best
Thank you bro I did watched one of ur videos for my last exam and I did very well
thanks bro very helpfull
Thanks my man, I actually learned this and now I'm able to do corrections. ;)))
Thanks so much!
does this guy not low-key sound like a supervillain?
Lol goddamnit
but he lowkey saves kids in science and math class everyday
@@murk.mp4760 until this day :D
Thank you.🥲🥹
great explanation
Absolute legend
Thanks a lot sir. ❤❤
after failing 2 cats and revising now i understand the concept thanks bro❤
Thanks
Thank you so much
Omg thanku so much
I love you man 😭😭😭
i love you! can we name you king of the universe?
thank you so much sir
Very good
If I keep a bucket of water which has vapour pressure of 2.34kPa @ 20°C, inside a closed room (ambient temp 20°C). It says water will keep evaporating until partial pressure is 2.34kPa for water vapour.
Does this mean that the total pressure of the room will increase?
Yes
Helpful for online classes
Thhhhhhhanks love u
In a gaseous mixture of CH4 and C2H6 there are twice as many moles of CH4 as C2H6. The partial pressure of CH4 is 40 mmHg. What is the partial pressure of C2H6?
what if 🤭🤧
🙏🙌❤️ much love always
I have a question
what if thgases combined together
then check how it reacts and find it,s final mole after reacting for ex N2 +3H2 gives 2NH3 now let's suppose all n2 combined to give 5 mole of NH3 and there is still 3 mole of H2 left but only H2 will not react with NH3 so we can use dalton law now
What if we removed on substance from the mixture, what would be the effect of that and how can we write in an equation ?
For partial pressure, we take non reactive gases. Reaction between N2 & O2 is endothermic; so, partial pressure laws are applicable for them. I think if we remove one substance, the remaining others will occupy the total volume of container and will have partial pressure accordingly.
we don't have to divide it by the total?? 6:37
like 2 mol+3 mol. to get the total like the first example??
then divide each by the total like.. 2/5 and ,3/5??
if you divide it by the total then you're solving for the mole fraction
ur goated to the max
COULD YOU ANSWER THIS PROBLEM PLEASE?
A certain reaction occurs, producing an oxide of nitrogen as a gas. The gas has a
mass of 1.211 g and occupies a volume of 677 mL. The temperature in the laboratory is
23°C and the air pressure is 0.987 atm. Calculate the molar mass of the gas and
deduce its formula. Assume the gas is ideal.
I am proud ❤
legend
Why torr is used for unit in pressure?
3:55 the Xo2 or oxygen, where did the 10 came from? the denominator 10? how- where- what-
10 came from the total number of moles of the gases.
n(Ar) +n(O2) + n(N2)
👇 👇 👇
5 + 3 + 2 = 10
Sorry if i responded late!
will be right back here after 10 years
wow this is easy and i havent done chemistry
so helpful
Thank You!!!!
In the last sample problem, Is it possible to determine the number of moles of the CO2?
#theorganicchemistrytutorslifematters to me
in the pv=nrt formula, doesn't V have to be in metre cube? why did you substitute in the value which had litres ?
how do you have videos on everything
If ideal gas equation is not applicable for any gas as they are real gases then why it is derived?????
Sir what if each gas is in a separate container and the mass ‘ temperature is given of individual gases but volume is not given
Last video then I'll gotta sleep in peace
YOO SAME
Hehe he same here gonna sleep just after this
just a little question isn't the universal gas constant 0.08314?
Hey in my textbook it says that the constant of R is 0.0821, is it the same or not?
0.08206 is more accurate, but 0.0821 works too. But, if you want a more accurate answer then stick with 0.08206 instead.
how to get the 0.08206..?
Lance Dela cruz You just plug it (0.08206 for R) into your equation. It is given to you, so you don’t need to find it.
Lance Dela cruz
PV=nRT
R=PV/nT
R=(1.0 atm)(22.4 L)/(1 mol)(273.15 K)
R=0.08206 (L•atm/mol•K)
The variables used were the conditions required to meet Standard Temperature and Pressure (STP).
0.08206 is a constant. Like Victor said, you just plug that number in.
Please can anyone help me abd tell me how get molar mass
molar mass is the equation drt/p (density*constant*temperature/ pressure) Hope that helps
love u bro, saved my ass
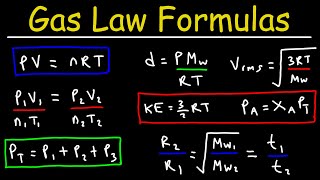
Partial Pressure 2:35
Since the question used 300K which is in Standard temperature, why did you 0.0821(R) instead of 8.315 in Standard pressure ?
I was wondering this too!
There are 2 of them, one for atm pressure which is (0.0821), and the other one is 8.315 which is the R for kPa. Its basically just difference in the units you use for your question. Sorry you probably know this by now because this is 3 months late lol.
@@danialbajwa8112 the question doesnt seem to specify a unit for pressure though, no?
R is a universal constant used in most chem problems, thus it does not change...u prolly know this by now
I thought the gas constant (R) was 8.314?
why do teachers make this concept so difficult?
i love you so much oh my god
I love you.
I need solution to this problem.
A sample of atmospheric air was found to contain 78% N2 , 0.03% of CO2, 16% O2, 1% rare gases,5% gaseous impurities at STP. If 200cm3 of the sample air was analyzed, calculate the volume of the respective component of the air hence calculate pressure 5 mole of the air will exert at STP.
how did you get 1000?
nvm just realized it
It's given in the question .
Total pressure 1000 turr
4:57
His voice is changed I think he was sick while recording this explanation :((
If h2 is collected over water at 22C and an atmospheric pressure of 100.8kPa, what is the partial pressure of the H2 when the water level inside the gas bottle is equal to the water level outside the bottle?
1:34 1:34
❤
This is where I give up
🙏🙏🙏
can someone get back to me asap for the second formula how do u find R to be 0.08206
It's a constant
Anyone from class 9th?? ☠️
I need to learn this if I wanna become a SEAL so🤷🏾♂️
Man, I cuckoo's love you
I hope I pass my supps🥺🙏🏾
gonna have exam 1 hour from now, will update wht happen
where did he get 32g and 28g?
lmao idk if Im too late but he had gotten those measurements from the molar mass of the elements so the molar mass of oxygen is 16 but since the subscript is alway 2 when alone (2)16 is 32g. Same for the other elements
@@carolynwoo8483 yeah I look at the periodic table and I got it. still thankyouuu ^_^