Molarity Made Easy: How to Calculate Molarity and Make Solutions
Vložit
- čas přidán 8. 09. 2024
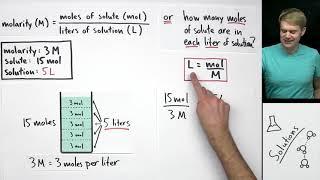
- Molarity is a very common way to measure concentration. It is defined as moles of solute per liter of solution.
Get $300 free when you open a Chase checking account with this code:
accounts.chase...
Offer expires 10/16/2024
Want FREE stocks and the ability to trade stocks for free?
join.robinhood...
The molarity formula is:
M = n / V
M is molarity
n is number of moles of solute
V is volume of the solution in liters
This equation may be rearranged to solve for any one of those three variables. The units of molarity are moles per liter, mol/L, or simply M, which is pronounced "molar."
In this video, I also describe how to make a solution of a certain molarity.
Please see my related video on mass percent concentration:
• Mass Percent of a Solu...
My goal is to make chemistry easier ;)
ketzbook.com
#mole #solutions #molarity #madeeasy #chemistry #ketzbook #tutorial