Henderson-Hasselbalch equation || Application and calculations
Vložit
- čas přidán 12. 06. 2020
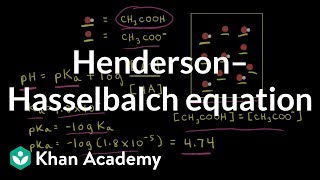
- Can we find pH of weak acid or weak base by knowing its concentration? Absolutely we can't as pH depends on its pKa and concentration of ionized form. Here Henderson-Hasselbalch equation comes into the play. So in this video, we have discussed significance of this equation along with few practical problems.
#phandpka
#henderson
#weakacids








sir please continue this series its very helpful
very detailed explanation sir thanks alot for the video
Great explanation, thank you
thank u so much.helpful vdo
That was a nice explanation
Awesome ❤️
Thx sir!
Sir,In gpat and Niper examination, do they provide logarithm book for calculating final ans?
As it is a online exam, they will not provide any logarithm book. You have to calculate on the rough paper given to you at that time.
For weak base POH = pkb + log BH+ /B …. I think
how to solve if we dont knw the mole of base?
like for example, how many moles NaoH to add to 0,040 mole of NaH2PO4 (Pka = 2), So we can get pH = 7,0 ??? please help me....
I am not an expert but I can try to help here.
The NaOH would react with the acid to form conj. base, which would be needed for the H-H equation.
*Reaction: NaH2PO4 + NaOH -> Na2HPO4 + H2O*
Using the H-H equation, with the goal in mind, I would get: *7.0 = 2 + log({mol NaOH}/{0.040 mol NaH2PO4})*
Since the weak acid and strong base are in the same amount of liquid, you do not need to calculate molarity.
Moving the 2 over, we get this equation: *5 = log({mol NaOH}/{0.040 mol NaH2PO4})*
Rearranging the equation (by using properties of logarithms): *10^5 = (mol NaOH)/(0.040 mol NaH2PO4)*
Dividing both sides by 0.040 we get: *100000*0.040 = mol NaOH*
To eventually get the amount of moles of NaOH needed: *4000 mol of NaOH.*
That seems ridiculously off but that's what I calculated, so maybe I did something wrong, however it seemed right when I plugged it in. The HA would be the NaH2PO4, and the A- would be Na2HPO4 (seems wrong, again, but this whole thing is wacky). I know this is late but I hope this helps!
How to calculate the ph of 0.005N HCL
You can use simply -log(0.005) which gives you a value of 2.3
Thank sir
How to calculate log and antilog value
Log value can be obtained from logarithm tables or simply by using a calculator. Antilog can be obtained by taking 10 to the power of x in calculator.
Thank you sir
Sir can you give some reference book on this topic❤🥳
So many references we can quote, but you can refer Vogel's textbook of quantitative chemical analysis. Thank you.
@@egpat thank you😁👍
There is major mistake I found while I watched this video. Value of pH in HENDERSON HASSELBALCH EQUATION is pKa +log [salt]/[acid] whereas value for pOH is pKa+log [salt]/base. in both equation salt is in NUMERATOR. But in this video pH value for weak acid mentioned salt in the Numerator whereas for pOH value salt mentioned in the DENOMINATOR.
You have mistaken. In the video it is given pH of weak base, not the pOH equation. For a weak base, when you calculate pH, salt comes in denominator. Even for a base, it is convention to measure pH rather than pOH. Hope it is clear. Thanks for watching.