Osmosis Animation and Experiments
Vložit
- čas přidán 25. 10. 2023
- Transcript:
Before we can talk about osmosis, let’s do a quick review about solutions. Solutions have a solute (like salt, or sugar) that gets dissolved in a solvent (such as water). OK, so now that we know what solutions are, check out this experiment.
This tube has a membrane right here that separates two solutions. The solution on the right has a higher concentration of solute and the solution on the left has a lower concentration of solute. Make a hypothesis: what do you think is going to happen?
Now, watch what happens over time.
Did you notice that the level of blue solution on the right went up and the level of solution on the left went down? Can you explain why this occurs?
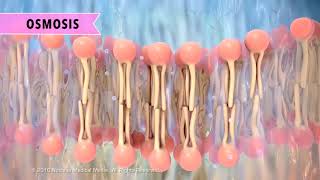
This happened because of osmosis! Osmosis is the diffusion of water across a semi-permeable or selectively permeable membrane, such as the plasma membrane that surrounds cells.
During osmosis, water diffuses down its concentration gradient, from higher to lower water concentration, just like other molecules or ions. You can also say that water moves from an area of lower solute concentration to an area of higher solute concentration.
Sometimes this confuses people, but if you think about it, it makes sense. For example, a solution with 1% solute is 99% water, but a solution with 20% solute is only 80% water. The higher the solute concentration, the lower the water concentration, and vice versa.
In our experiment, water moved toward the area with the higher solute concentration. Here’s an easy way to remember the direction that water will flow during osmsosis: Water follows solute! If you can remember that, you’re basically all set!
But, we do use a few terms to describe relative solute concentrations that you need to know.
1. HYPERTONIC: If we are comparing 2 solutions, the area with the higher solute concentration is HYPERTONIC. Water will always flow across a membrane toward the hypertonic solution! Remember, water follows solute!
2. HYPOTONIC: This is the solution with a lower solute concentration. Water will leave a hypotonic solution by osmosis.
3. ISOTONIC: If two solutions have the same solute concentrations, they are isotonic. The amount of water moving between isotonic solutions is equal so there is no net change in water amounts between the two solutions.
Now, let’s look at some real blood cells undergoing osmosis. These cells are in a hypotonic solution. Make a hypothesis: What do you think will happen?
The cells popped because water moved into them by osmosis. The cells cytoplasm has a higher concentration of solute than the distilled water that they are floating in, so water rushed in and caused them to explode!
Now, let’s look at the opposite effect. Here is an egg that has had its shell removed by being soaked in vinegar overnight. We are going to soak it in corn syrup, a HYPERTONIC solution. Look at how thick the corn syrup is! That is because it is very concentrated with sugar, a solute. Let’s speed this up. Watch what happens as the egg soaks in the Hypertonic corn syrup. Notice how it shrinks and becomes shriveled up, like a raisin. This is because it loses water due to osmosis!
To practice more with osmosis, check out the interactive experience at biomanbio.com, linked in the description. If you are enjoying these videos, please feel free to like and subscribe.
Blood Cell Video By M. Grundner, U. Klančnik & J. Derganc, Institute of Biophysics, University of Ljubljana, Slovenia: www.youtube.com/watch/OYoaLzo...
Used with permission: Creative Commons Attribution license (reuse allowed)







How does a 4 minute video explain more than my teacher in a whole week
I'm glad it was helpful to you!
Because they gotta teach it to 25+ students multiple times a day who don't care enough to watch this video like you did 🙂
Your teaching skills clear all my doubts amazing Sir nice
@@Prince20618 Thanks for your comment! I'm glad it was helpful!
you are the best biology teacher i've ever seen thank you so much for your effort and please upload more oftenly WE NEED IT! thanks so much
Thanks, I'm glad the videos have been helpful to you! I'm definitely planning to make more! Please feel free to subscribe if you'd like to be notified when they come out. Thanks!
dw bio man i am already subbed and i always like ur videos and i also made ur notifications on always :)
@@BioManBiology
@@spxken Thanks, I appreciate that! All the best to you!
Wth "water follows solute" just solves everything!!
Tysm ❤
I'm glad it helped! Thanks!
@@BioManBiology yeah it's absolutely perfect, I wish my teacher was like you!
Just wondering: I think it would be great if your channel expanded beyond Biology and maybe into Chemistry, you seem to be really great at that too!
Thanks, I am actually hoping to get into other disciplines eventually, but it is taking a while since I'm also teaching most of the day. Hopefully soon!
@@BioManBiology Oh you're a teacher? That's great your students are so lucky to have you! I'm in 8th grade and my Bio teacher recommended your Photosynthesis video and that's how I found you. Keep up the good work. :)
Thanks for your kind words!
So helpful. I was a bit confused about the solute and solution part but now its clear.Thank you ❤
I'm happy to hear that! Thanks for your comment!
That was a great way of explaining osmosis thank you!!
Thanks, I'm glad you enjoyed it!
thanks for this explanation, you're a hero BioMan !!
😄 Thanks! I'm glad you enjoyed it!
Amazing examples. Thank you!
Thanks, I'm glad you enjoyed them!
Thank you so much Bioman! This video really helped me with my biology test that had osmosis. God bless love from Pakistan ☪️🇵🇰💚
You're welcome! I'm glad to hear that it helped you on your exam!
Hey, you're super inspiring. Thank you 🫶
Thanks, I'm glad you are enjoying the channel!
Can't wait for the next video! What will it be about?
Thanks! I'm working on a digestive system game right now and I'm considering doing a video to go with it. I have a bunch of other ideas too but I'm not sure which one I'm going to go with...
Very clear explanation - thank you! Will use this in class!!
I've been looking for something like this for awhile - this is perfect!!
I'm glad to hear that! Thanks for your feedback!
Thanks, I'm glad it is useful for you!
Excellent explanation!
Thanks!
Best teacher ❤
Thanks, I'm glad this was helpful!
vaary vaary GOOD video sirrrr
Thanks, I'm glad you enjoyed it!
I have a question about cellular respiration. Is there a difference between fermentation and anaerobic respiration? Becuase I have heard that Anaerobic respiration is like aerobic respiration, but with a different electron acceptor at the end of the electron transport chain. But I’ve also heard from a lot of people, that fermentation and anaerobic respiration are the same thing.
Good question. Many people do use the terms as synonyms, but technically they aren't the same. This article does a good job explaining the difference I think:
www.khanacademy.org/science/ap-biology/cellular-energetics/cellular-respiration-ap/a/fermentation-and-anaerobic-respiration
It's basically what you described, but in most introductory courses that I've seen, fermentation is usually described as anaerobic respiration. So, you might want to check with your teacher about their expectations on this. I hope that helps!
@@BioManBiology Thank you, this really helps! Seems to me by the article that fermentation is an anaerobic pathway, but not a type of cellular respiration(or anaerobic respiration). I will definitely ask my teacher, as I feel the terms might get mixed up on our tests. Thanks for your help!!😁
Great❤❤❤
Thanks a lot dear❤
Thanks for your feedback! I'm glad you enjoyed it!
Loved the video.
Thanks, I'm glad to hear that!
Can you share where you purchased the u-tube apparatus? I have not been able to find it anywhere and think it would be really useful for some simple experiments.
I believe I bought it through Pasco. It is a cool apparatus, but the process is pretty slow. The video shows an overnight time lapse. Best of luck!
0:54 - 1:25 : "Osmosis is the diffusion of water across a semi-permeable or selectively permeable membrane, such as the plasma membrane that surrounds cells.
During osmosis, water diffuses down its concentration gradient, from higher to lower water concentration. Just like other molecules or ions.
You can also say that water moves from an area of lower solute concentration to an area of higher solute concentration.
Sometimes this confuses people, but if you think about it it makes sense."
It's purely magnificent as a definition of osmosis, because in French the definition of osmosis seems to make water pass as a molecule which does the opposite of diffusion (the opposite movement) but now I understand well that this is not the case, it is just that one of the definitions of osmosis (surely the most common) describes the movement of water in relation to solutes! Thank you very much and infinitely! 🙏🏽
I'm glad you found the explanation helpful! Thanks for your comment!
Thanks really helped!
Glad to hear that! Thanks!
thank you
You're welcome!
Hello sir thanks for the video. Is the reason why water wants to flow to higher solute concentration because dissolved substances such as salt, sugar applies a "force" to water molecules which cause them to be attracted? I want to understand "physics" of the topic but no source proivdes fundamental physical reason of why water flows there. Thanks
I'm glad you enjoyed the video! Here's an explanation of the physics of osmosis from the Journal of General Physiology: www.ncbi.nlm.nih.gov/pmc/articles/PMC10457415/
It goes into a lot of detail. Apparently, the physics of osmosis is pretty complex 😉. I hope it is helpful to you.
what membrane that you use? is it availble at general store?
I think I got the apparatus and the membranes that fit it from Pasco (a science supply company), but I'm not completely sure because I got it a while ago. I hope that helps!
@@BioManBiology What type of membrane please?
I hope you look for me for his type I would be very grateful
So what is osmotic pressure? Is it relate to osmosis
This site has an explanation that you might find helpful: kids.britannica.com/students/article/osmosis/276223
All the best!
Got a test today yall 🙏🙏
Good luck on your test!
I have a doubt. Why books teach us osmosic happens high to low concentration in india? Plz clear my doubt
My guess is that the book is teaching that water moves from an area of higher water potential to an area of lower water potential. This is similar to saying that water moves from an area of high water concentration to an area of lower water concentration. Notice that a higher concentration of water is a lower concentration of solute and a lower concentration of water is a higher concentration of solute. The confusion arises because we often talk about the solute concentrations of solutions.
For example, a hypertonic solution is high in solute (but low in water), so water moves into it (from high water concentration to low water concentration). I hope that helps!
What type of membrane please?
This animation is of a generic cell membrane, also called a plasma membrane.
Please make video on female reproductive system (I find zoology a bit difficult) you are my favourite bio teacher!!! please!!!
Thanks, I'm glad you are enjoying the videos! I'm not sure if I'll be able to get to the reproductive system anytime soon, but I am planning on making videos for some other body systems soon!
@@BioManBiology Thank you so much for replying!! You made my day ;)
tysm man
You're welcome!
2:10 Bro you use both the same solution (sugar in water), so its should be called "hyperosmolar" instead of "hypertonic".
Hyper/hypo/iso-tonicity are used to compare onr solution to another type solution (like sugar solution to salt solution). While hyper/hypo/iso-osmolarity are used to compare the same type of solution (sugar solution with another sugar solution).
CMIIW
cheers bud
Cheers!
Cheers!
❤❤
Thanks!
An egg shriveled up like a raisin is for some reason repulsive
This explains nothing. *WHY* is the water flowing like that at all.
This is like saying electricity flows from negative to positive, and just leaving it at that, there's a *reason why and it's important, it's because the electrons r that what is moving and they* are attracted to the positive terminal,
So...
Why* the hell is the water moving from low concentration to high concentration?
I want a complete answer and I expect u to show ur work. 😊
Free Palestine (Raise awareness about human rights violations in Gaza)
Shut up
How does that have anything to do with a science video
@@balkanboys768 It doesn't, it just reminds everyone that there are people that need our help.
Don't bring your politics into these unrelated science videos.
@@plukovnik-skvelous Say that to the bodies of the 12K brutally murdered children.
Thank you for this informative and clear video. You're the best, Bioman! 🤍
Thanks for watching it and for giving me feedback! It is much appreciated!