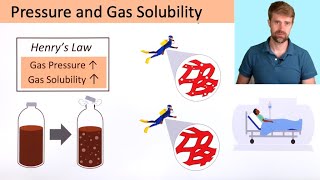
Henry's Law and Gas Solubility Explained
Vložit
- čas přidán 22. 11. 2016
- www.thegreatcourses.com/course...
I am Professor Davis, and in this short clip I explain how Henry's Law can be used to calculate the solubility of a gas using its partial pressure and its Henry's Law constant.
This clip is just a short sample from my new 60-lecture course available from The Great Courses. Learn more about the course at the link above and learn more about me and my other projects at / chemsurvival








The only explanation that's helped me understand Henry's Law. Much love from Washington!
Yo, a recent comment!
@@samuraijosh1595 hello from the future! buy NVDA !
Thank you. Great explanation
When beer and soda peopled talk about the "volumes of CO2" in their beverages, what exactly do they mean? Based on this video, I would say that a beverage under 4 atm of pressure (or 59 psi) at room temperature would have 2.7 liters of CO2 (at STP) dissolved in it for every liter of water. That sounds like 2.7 volumes of CO2 to me...but my "PSI to Volumes" charts say differently. So what is the right definition of "volumes of dissolved CO2"?
Well said. It happens my name is Henry and I love the science of absorption, adsorption, phase change, energy transfer etc because of my work with Hempcrete. Would love to start a conversation with you.
This was helpful.
How did you get the new pressure for CO2, 4x10^-4 atm?
It's the partial pressure of CO2 in air. About 400 ppm.
@@user-qn9ym6hz2c how did he came up with22.4?
@@sofiasirajsirajabdu4258 that's a standard constant which is apply in 0 degrees c (STP), and 24.4 for 25 degrees c (RTP)
Can any one answer what is the relationship between the Henry's law constant and CO2 and affect on PH.
what about temperature? is that not in the equation?
thanks for such a lovely explanation. love from India
what could be the reason for the exclusion?
Thank you sir. Much love from india❤️❤️
Very helpful to clear my concept .......... from a student of class XI
Nice Expanation
Thank you for this explanation. I'm confused why they call it "dissolved" which usually refers to a solid dissolving into a liquid changing its physical structure. it just seems that it is only referring to the presence of CO2 (in its gas form) in the liquid, which needs a considerable amount of pressure to force it into that liquid anyway. So does its structure/physical properties change in any way when described as "dissolved" or is it still in its gas form? I'm confused as it seems to contacdict the physical phases graph of CO2 to refer to it as "dissolved". Apologies if this is a stupid question.
if you matched it one co2 for every h in the water, the co2 would probably class as a solid now u compressed it down so much. i think its about 20,000 psi to do it. (9-10 tonnes of pressure.)
Thanks for video
Assuming the solubility of CO2 in water when the can opens was not negligible, would we subtract that amount from our final answer? Also, where did you get the partial pressure of 4.0*10^-4 atm from? Thanks! :)
Partial Pressures of Atmospheric Gases table... 0.3mm Hg = 3.9 x 10^-4
Useful
Just leave us hangin like that ?!
Yes, this is actually an ad and doesn't have the honesty to say so at the beginning. He didn't even talk about a partial pressures.
Veryy helpful.
Love from India.
I'm so glad it was useful to you! Love back from America.
Why does it drop to 4.0 * 10^-4 atm? The standard atmospheric pressure is 1 atm and not 0.0004 atm.
.0004 atm represents the amount of CO2 in the atmosphere as a partial pressure. All the atmospheric gases act independently and add up to 1 atm.
There is 0.04% CO2 in the atmosphere (approximately). So according to Daltons Law of partial pressures, the partial pressure of CO2 should be (mole fraction or fraction of molecules)*(total pressure)= (0.04/100)*(1 atm) = 0.0004 atm
Where’s did we get 0.04% now. From the statement we have 4atm Pco2.
Is there a law or formula to calculate what time is required for the dissolved gases to affervate (come out of the liquid) once the pressure is removed???
No
I think Graham's law of diffusion or effusion will help you indeed which states that rate of effusion is directly proportional to pressure/square root of molecular mass.
And well that's a comparative law! You can use to compare two different effusions or diffusions!
Hope this helps :)
Very interesting
The lecture was amazing but it would have been even better if you discussed mole fraction relation .
Keep it up sir 🙏
Where is the 22.4 L/lol derived from?
PV = nRT
When:
P = 1 atm
n = 1 mol
T = 273 K
R = 0.082 L*atm/(mol*K)
V must be equal to 22.4 L
ChemSurvival But the problem says that it's room temperature (25°C = 298,15K).
@@MrAntsunator Hi, *Note that In PV=nRT, both side the unit of temperature is in Kelvin, so it doesn't matter weather the temperature is in Kelvin of Celsius. If 25°C = 298.15K then R must be in Celsius also...* I hope you understand that...
Thanks sir
Where did you got the value of k
Rudra sai prasad it’s a constant....you have specific value for each gas
Very helpful thanks for this video 💖 from INDIA
Hat
@@pavansuman2600 tu hat
thanks a lot
This may help us solve risigg sea temps and sea acidification
Thank u sir
Thanks for the video! But isnt it true that CO2 doesnt obey Henrys law???
You're correct. The CO2/water system is a particularly non-ideally behaving system when it comes to Henry's law and maybe wasn't the best choice for an example. This is largely because of the complex equilibria among dissolved carbon dioxide, carbonic acid bicarbonate and carbonate ions that results when CO2 is dissolved in water. In hindsight a different gas or a different solvent might have been a smarter choice. Nonetheless, the fundamentals of Henry's law are well covered I think.
Love from India 🖤🖤🖤
From a jee aspriant 🖤🖤🖤
killed it
I have good idea to increase solubility of gas in water without increase pressure...
what is this idea.i wanna get a wider understanding of this topic.
another point can we be whats friends.
@@vevo5086 surface of water and area of gas is too high... so it is required more pressure for solubility... So we divide it to very small particle... Its up to 300 - 500nm tube for water... 2000-2500 milli litter for gas... We can achieve this... Definitely we increase solubility of gas...
Henery's law is P=kS and not what stated in the the video.
Write Brother kya aadami h sala
Thanku sir😍 I'm from India🖤
You are very welcome!!!!
@@ChemSurvival thanku 🖤 so much sir again
Badiya hai mere pass😊
Hey
hi sheldon
Easy to understanding love from India I am a jee aspirents
Hello
zup
Rajkiran Sir Rocks
could you give us the weather report while you're there?
Partly cloudy with a 100% chance of the partial pressure of a gas being proportional to the concentration of that gas dissolved in the solution below it...... duh
ChemSurvival 😂😂
@@ChemSurvival 😂😂👍👍👍
Just proved soda gets flat over time once opened
there is a problem in this answer
he looks like a freakin meteorologist on TV loll
Today's prediction... 100% chance of you subscribing to my channel B-)
Soda.
Never trust anyone that does scientific notation for .12.
That dramatic music at the beginning....really???
Just having a little fun with Movie Studio.... Don't be hatin' :-)
ChemSurvival uhhh...im not
good to hear (and I hope you liked the rest of the video more than the intro). Plenty more videos on my channel that I hope you will check out, also.
Yes it was necessary