IPTG induction using the lac promoter
Vložit
- čas přidán 5. 09. 2024
- The basic principle behind recombinant protein expression is that we can stick the genetic instructions for a protein we want made into cells from a different organism and it’ll make the protein for us. We can steal a clever biological setup from a bacteria-infecting virus (a phage) - the LAC OPERON, to be able to control when we express the protein, so we can allow the bacteria to grow lots before we get them to focus on protein-making and not dividing. All we have to do is stick a lac promoter in front of our gene of interest, then add IPTG when we want the cells to make protein. This tricks them into thinking lactose is present and they need to make lactose breakdown machinery.
Much more detail here: bit.ly/bacteria... ; longer video with more on the T7 system: • Inducible protein over...
But here’s the just the gist version… • IPTG-inducible protein...
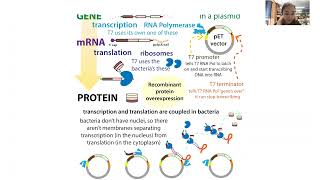
A gene (DNA recipe for making a protein)’s “natural home” is in a chromosome, which is a really long strand of DNA that holds a lot of genes. We’re only interested in one (and we want the edited version - the DNA copy of the mature mRNA, which we call complementary DNA (cDNA). So, using methods like “cutting and pasting” with restriction enzymes and DNA ligase or “copying and stapling” using PCR-based methods like SLIC, we can stick the cDNA for the protein we want made into a smaller piece of DNA that’s easier to work with and which has some special features. We call this carrier DNA a “vector” and for bacteria, the vector is usually a small circular piece of DNA called a plasmid.
Bacteria use the LAC OPERON to control when they make the machinery for breaking down the sugar lactose. They only want to make that machinery if there’s lactose present, so when there isn’t, a repressor protein (LAC REPRESSOR) sits on the LAC PROMOTER site where RNA Pol needs to bind & “hides it” Then, when lactose is available, some of that lactose gets converted to allolactose which binds the repressor. This causes the repressor to change shape & fall off, freeing the promoter for RNA Pol binding
If we stick a lac promoter in front of our gene & don’t give the bacteria lactose (it’d rather eat glucose anyway) the T7 promoter will stay hidden, so none of our protein will be made. I say “no” but the promoter can “leak” if the repressor falls off on its own and RNA Pol sneaks in before it rebinds. So, for tighter control, we can stick do things like use the lac promoter to control expression of T7 Pol & use a T7 promoter in front of our gene of interest.
Instead of adding lactose or allolactose, which the bacteria can break down, we add the allolactose mimic IPTG (Isopropyl β-D-1-thiogalactopyranoside), it binds the repressor ⏩ repressor falls off ⏩ RNA Pol binds promoter in front of our gene ⏩ RNA Pol copies the DNA into RNA ⏩ does this over & over 🔁 making lots of mRNA copies that swamp out the bacterial mRNA & outcompete for the limited ribosomes ⏩ ribosomes make our protein from the mRNA instructions ⏩ we celebrate!
Well, sometimes we celebrate. But sometimes we’re not so cheery because sometimes they make too much for the cell to handle, so the cell can’t fold our protein properly & the protein forms clumps of aggregates called inclusion bodies, and when we break open the cells (lyse them) to get out our protein and then spin them down (centrifuge them) to pellet out the insoluble stuff like membrane bits, and we expect our protein to be in the liquid part, its actually with a bunch of crud in the pellet. BUT, all hope’s not lost - we can try again & lower expression by reducing inducer concentration (add less IPTG) and/or growing at a lower temperature.
But sometimes that’s not enough to get you the protein you want. It’s easiest to explain recombinant protein expression in terms of bacterial expression systems, and a lot of proteins are expressed this way (probably most of them) - but some proteins don’t express well (or at least they don’t survive the expression process well) in bacteria - because even though bacteria have all the copying machinery, they don’t have the same folding helpers and post-translational modifiers our cells do - so they can misfold & clump up, have different phosphorylation (added phosphates) & glycosylation (added sugar chains) patterns
So for these trickier proteins we can express them in cells more like ours - mammalian cells are harder (but doable), but insect cells like Sf9 aren’t too bad. I expressed a lot of my proteins using those in the past. finished in comments








Amazing Video thanks a lot
Most welcome - glad it helped!
But when I can use bacteria, I do because it’s way cheaper & easier - and - when it works - you can get a lot more protein per liter. They have really simple growth conditions - they grow fastest at ~37°C, so we set the shaker incubator thermostat to this nice warm temp when we want them to grow and multiply lots. The shaking is important because it makes sure the cells stay aerated - each cell gets a chance to be closer to the oxygen, and CO₂ doesn’t build up - for proper aeration you need to leave a lot of empty space in the flask (like at least 3/4 of what the flask says it holds). I do small “starter cultures” overnight (50ml) so I can get a lot of cells to start with. Then I add some (usually ~5ml) to 1L portions of media in 4L flasks.
Now I have to start monitoring its growth - I want them to grow enough that I get lots of cells (my “factories”) but I need to make sure each of these factories gets enough supplies & doesn’t have to compete with one another for resources. So I periodically check the OD600 to tell me how dense the media is which (the more cells there are the harder it is for light to pass through it) and we can measure this cloudiness as the “Optical Density” measured by a spectrophotometer that shines light (in this case light with a wavelength of 600nm) through a sample of it in a little square “tube” with clear walls called a cuvette and measures how much of the light makes it through.
What’s the optimal optical density for induction? It’s protein - and media - dependent. For LB (Lysogeny Broth) I normally aim for ~0D 0.6-0.8. TB media is more nutrient rich, so it can support denser cell growth - I usually aim for an OD600 of ~1.4-1.8. Once I see it getting close, I move the flasks to the cold room and decrease the incubator temperature to 16 or 18°C.
I typically add IPTG to 1mM but the optimal amount is protein-dependent once again. When I add IPTG, mRNA for my protein starts getting made. And then the ribosomes start making protein from it. I let them make protein overnight at that 18°C temp - at this lower temp protein making’s slower which gives proteins more time to fold the right way and hopefully prevent aggregation.
In the morning, I can “harvest” the cells by pouring the liquid holding them into bottles, centrifuging them (spinning really fast to pellet them out cuz they’re heavier than the liquid), re-suspending them in a bath of nice clean buffer (pH-stabilized salt water), then breaking them open (lysis) and purifying out my protein - which is made “easy” because I’ve used DNA Pol to help me redesign the gene to add a little tag onto the end that will specifically bind little beads (resin) in affinity chromatography.
more on troubleshooting if your expression isn’t going well: bit.ly/wherestheprotein
more on bacterial growth media: bit.ly/bacterialmedia
more on molecular cloning: bit.ly/molecularcloningguide
more on bacterial strains: bit.ly/bacterialcelltypes
more about all sorts of things: #365DaysOfScience All (with topics listed) 👉 bit.ly/2OllAB0 or search blog: thebumblingbiochemist.com
#scicomm #biochemistry #molecularbiology #biology #sciencelife #science #realtimechem
Does too high OD affect the expressed protein in TB? I've learned that too high OD might push the expressed protein into inclusion bodies. Or this is just the case for LB?
except in my lab we used IPTG to induce the expression of an shRNA instead of an mRNA. As long as you designed it that way when inserting the DNA into the plasmid and ligate then you can do that right? also to get that into our mammalian cells i think they did transduction. Does that sound right?
transduction is if you are using a virus to introduce genetic information - the more generic term for getting genetic info into cells is "transfection" - because the lac operon works at the level of transcription you can use it to induce transcription of different types of RNA
and can you make a video on DOX induction?
maybe at some point, but I haven't done that before
also what is the difference between the 2 videos you have on this?
not sure which 2?