Potential Energy Diagrams - Chemistry - Catalyst, Endothermic & Exothermic Reactions
Vložit
- čas přidán 13. 07. 2016
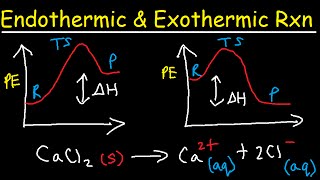
- This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the forward and reverse activation energy. It describes the relationship of the enthalpy of a reaction with the potential energy difference of the reactants and products. It also shows you how to identify the transition state or activated complex as well as any intermediates. This video shows you how to draw a 2 step PE diagram and a 3 step potential energy diagram. In addition, it shows you how to identify the slow step or the rate determining step.
Chemical Kinetics - Initial Rate Method:
• Chemical Kinetics - In...
Rate Constant k - Find The Units:
• How To Determine The U...
Integrated Rate Laws - 1st & 2nd Order:
• Integrated Rate Laws -...
Reaction Rate Factors:
• Factors Affecting the ...
Collision Theory & Activation Energy:
• Collision Theory - Arr...
___________________________________
Potential Energy Diagrams:
• Endothermic and Exothe...
Elementary Rate Laws:
• Elementary Rate Laws -...
Rate Laws of Reaction Mechanisms:
• Writing Rate Laws of R...
Intermediates & Catalysts:
• How To Identify The In...
Types of Catalysts:
• Homogeneous vs Heterog...
____________________________________
The Equilibrium Expression:
• How To Write The Equil...
Calculating Kp From Kc:
• How To Calculate Kp Fr...
Chemical Equilibrium & Ice Tables:
• Chemical Equilibrium C...
Le Chatelier's Principle:
• Le Chatelier's Principle
Acids and Bases - Introduction:
• Acids and Bases - Basi...
______________________________________
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tutor.net/chemistry...








Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Final Exams and Video Playlists: www.video-tutor.net/
this man has a spot reserved for him in heaven
Yea this guys the man
Ashley Jung fr
Facts
not yet
Bhol jao
I can't comprehend how he makes the most difficult subject the easiest to understand. This man truly deserves the world.
He should make a video on how to be an effective teacher so he can explain that to us as well.
This guy is the sole reason I’m doing good. My teachers are very very intelligent but he makes things very simple, takes his time. My teacher has notes pre-made and still takes 80 mins to explain what he does perfectly in 8-15 mins.
Truly a legend
YES! I finally understand it now just hours before my unit final!!!
me: *casually panicking because I don't understand my work*
this beautiful person: I got you *proceeds to help with all my life problems*
This man is responsible for me surviving both Chem and Calc II. You are the best JG
This is so helpful. You're the only reason I understand anything going on in my chem class!! Thank you so much!!
07:25. LOLed at "This is the intermediate, which is like, in the middle"
I wish my professor taught like this; straight to the point and not over explaining to confuse students
really good lectures, this guy knows his stuff..
Thank you for being a good teacher when I needed one
All the ones we have can't seem to explain things in a simple manner and get frustrated when we're confused. As if they're job is not to teach and enlighten
About to take my chem lab exam and the professor made it known that there will be an energy diagram for sure... thanks for the great review!!!
God bless this genius man! You have really helped me a lot!!
You are the only reason why Im going to do well , thank you Soo much
Thank you sooo much for making me understand about these diagrams.
the GOAT account for chemistry man THANK YOU!
You just saved my life, thank you!
It’s like he knows all the questions i had ...👏🏿
Very helpful! Thank you so much
The GOAT of High school and AP chemistry
It all makes so much sense now. Thank you very much. 😊
smh his voice is so soothing i fell asleep
very nicely explained thank u
Thanks, my chem teacher (normal chem) isn't teaching us anything because she dumbs down the class for the people who don't care about their grades. This is actually pretty helpful. I learned more in this video than we did in a 45 minute class period where she was saying the same stuff over and over. The way you explain these topics is simple and time-efficient. My teacher didn't even tell us what enthalpy was, merely that we should see it and think "change in heat" (because delta-H). Thanks for the help.
Very clear explanation, much appreciated
tysm!!! u explain so well
Love this guy
Your lessons video are really helpful
THANK YOU SO MUCH. I AM SO GRATEFUL T HAVE YOURS VIDS.
I found out your explanation is waaaay easier understanding process than my teacher at the school! When they just give me big thick text book read through the equation process.
Thanks man really helped me with my presentation
Best video ever!!! Thank u! I was so worried.
Thanks a lot.I am grateful to you.
very helpful ❤
super helpful!
Your a saint that was amazing thank you !!
Really helpful 💯♥️
Thank you!!!
Spot on JG >U r breathtaking
Helpfull 100% thx
Thank you so much 🥰🥰🤗🤗
Best chem teacher ever
Thank you you are best
Many thanks.i love ur channel
Nice I love it
Thanks a lot.🤗
07:56 it's a sign from God "R I P"
Nice lecture
thank you so much
You are my hero.
You are genius! Thank you!
I love you, tysm
Thank you so much you saved my grade
Joey Timp bro same
so I have a question where im given a graph like shown, with multiple numbers that are all amount of Kj and im told to right the chemical reaction of A + B = C
please explain
5 years later still helping people pass exams
thank you THIS REALLY HELPED, I HAVE A CHEM FINAL TOMORROW AND KNOW NOTHING
How did you know I have a exam in 2 weeks
@@kalsang6255 Summer semester?
YOU ARE AWESOME
very nice !!!!!!! reallyy
bro out here saving lives
Thx dude
Can you answer this question? Do these diagrams show Internal energy or just the chemical potential energy? From thermodynamics I know that it's the change in Internal energy (without pressure or volume change) that equals heat exchange.
Hello, Do you how the potential energy diagram of hydrolyisis of acetate of methyl looks like?
Thanks 🙏🏿🙏🏿
ly
broski
best tutor
zo helpful vedio
Good
Super helpful I mean damm and holy s*** 2.81M sub bro this guy awesome!!!
On God you're my hero ur in my prayers bro
U are a great being love u
Awesome
I love you bro
Are you available for tutoring?
how do u know the energy difference is completely driven by enthalpy and not entropy?
Fabrizio & this guy🫂
Your vidio so good but can you translate in Indonesian because it will help me to understand, i am verry grateful
pls can we also find the change in enthalpy of an exothermic by subtracting the product directly from the reactant
yeah
What will be the activation energy of intermediate steps
If a catalyst was applied to this, would it lower the activation energy of only the RDS?
I don't know man, I don't know 😭
how do we know if something reaeases high energy?
lmao watching this an hour before I take my Exam lol
Fire
How you come to know that the products have more energy than the reactants?
Why does not C react with oxygen at room temperature ? Is that because it has high activation energy ? 😯
Is it product minus reactants or reactants minus products?
Is enthalpy change the same as change in energy??
I'm confused between these and would appreciate an explanation.
Thanks:)
Its always the Final - Initial... So, products minus reactants
Tysm my bio teacher can’t teach
Yo Mark Wahlberg is secretly a chemistry tutor??
Thank y🤰🏻
*Sir please please 🙏🥺🥺🥺🥺🥺🥺🥺🥺 give me the diagram of kinetic energy*
This is very easy I just can't CONCENTRATE in class
But if it's youtube, you can concentrate haha.
HELP WHAT ARE THE UNITS ON THE AXISES IM CRYING PLEADE
if this isn't love then what is
Two step reactions statrt from 7:05
Thanks
how do you figure out how much energy they contain tho
How much energy what contains?
7:15
You taught me more than my stupid chemistry teacher has in the past week
Good night
Can you replace my current Chemistry teacher?
Gg
Wassup Cayabyab kids